

 2021-09-02
2021-09-02
Abstract
Objectives. We tested the hypothesis that pravastatin (PRA) activates endothelial nitric oxide synthase (eNOS).
Background. Pravastatin has been found to have clinical benefits beyond those predicted by its actions in reducing plasma low density lipoprotein cholesterol (LDL). Both PRA and simvastatin (SIM) are equally effective in reducing LDL, but only PRA reduces platelet aggregation and is an effective vasodilator. Nitric oxide (NO) also inhibits platelet aggregation and vasodilates.
Methods. We determined PRA and SIM effects on vasorelaxation in aortic rings and NO production by cultured bovine aortic endothelial cells. Nitric oxide was measured by using a NO electrode and by an assay for conversion of hemoglobin to methemoglobin. Specificity of NOS activation was tested by using the NOS inhibitor nitro-l-arginine methyl ester (l-NAME, 1 mmol/liter) in the presence or absence of excess l-arginine (l-ARG, 1 mmol/liter).
Results. Endothelium-dependent vasorelaxation was maximal with acetylocholine (ACH, 100%), followed by PRA (62.8%) and then SIM (37.1%). Direct measurement of NO confirmed that vasorelaxation is due to NO release and showed that PRA and ACH had similar dose-dependent effects on NO production, while SIM was only 25% to 30% as effective. Methemoglobin assay confirmed these results and demonstrated their specificity for NOS activity. The l-NAME blunted the responses to 45% of initial values. Excess l-ARG reversed this effect and potentiated NO production to 133% of initial levels.
Conclusions. Both PRA and SIM activate eNOS, but SIM is much less effective. Clinical benefits with PRA not explained by LDL reductions may be the result of an independent action of PRA on eNOS activation.
Abbreviations
ACH
acetylcholine
BAECs
bovine aortic endothelial cells
eNOS
endothelial nitric oxide synthase
l-ARG
l-arginine
LDL
LDL cholesterol
l-NAME
nitro-l-arginine methyl ester
MetHb
methemoglobin
NO
nitric oxide
PRA
pravastatin
SIM
simvastatin
Clinical trials with pravastatin (PRA) have demonstrated reductions in myocardial infarction (MI) and stroke beyond those predicted by reductions in low density lipoprotein cholesterol (LDL) (1). Similar benefits were seen in patients without hypercholesterolemia following MI (2). Thirty-two patients with stable coronary disease treated with PRA and simvastatin (SIM) had comparable reductions in LDL, but inhibition of platelet thrombosis was seen to a much greater extent in the group receiving PRA (3). Studies in cynomologus monkeys fed atherosclerotic diets revealed that the arteries of the PRA-treated group had better vasodilator function and plaque characteristics more consistent with stability than did those of monkeys not receiving PRA. The beneficial effects of PRA were independent of changes in LDL (4). Finally, hypertensive Dahl salt-sensitive rats receiving PRA had blood pressure reductions and renal protection by an unknown mechanism that is unrelated to changes in LDL (5).
Pravastatin differs from SIM and the other statins as a result of its open lactone ring chemical structure. When administered as the active acid, its unbound plasma concentration available for distribution to the peripheral circulation is approximately 100 times greater than that of the open-ring form of SIM (6). Other studies have established that less than 5% of an oral dose of SIM reaches the systemic circulation as the open hydroxy-acid form (6). Therefore, PRA unlike SIM and the other statins has the potential to interact with the endothelium in a unique manner and this may, in part, be involved in the benefits seen with PRA that are beyond those predicted by LDL reductions.
Nitric oxide (NO) is a vasodilator and potent inhibitor of platelet aggregation with actions on the IIb-IIIa receptor (7). Some drugs [angiotensin-converting enzyme inhibitors (8), amrinone (9), amlodipine (10), nebivolol (11), s-nitrosylated tissue type plasminogen activator (12)estrogen (13), heparin (14)and insulin (15)] possess an auxiliary mechanism of activating endothelial nitric oxide synthase (eNOS). The purpose of our study was to test the hypothesis that the unexplained clinical benefits and actions of PRA result principally from an auxiliary mechanism of eNOS activation.
Materials and methods
Drugs and chemicals
The following materials were used: nitro l-arginine methyl ester (l-NAME), Earle’s balanced salt solution, acetylcholine chloride (ACH), oxyhemoglobin, pepstatin A, leupeptin, bestatin, phenylsulphonylfluoride, and l-arginine (l-ARG). All were purchased from Sigma Chemical Co. (St. Louis, Missouri). Pravastatin (PRA) and simvastatin (SIM) were of pharmaceutical grade.
Both PRA and ACH were dissolved in cell medium or Kreb’s solution. Simvastatin was prepared in two forms. The native, closed-lactone ring form is sparingly soluble in water. Thus, it was dissolved in dimethyl sulfoxide (DMSO, 0.2% as stock). To open the lactone ring, 4.2 mg of SIM was dissolved in 0.1 ml of 95% ethanol and then 0.15 ml of 0.1 N NaOH was added. After heating at 50°C for 2 h, the resulting solution was neutralized with HCl to a pH of approximately 7.2 and brought up to a volume of 1 ml with distilled water. Stock solutions (1 mol/liter) were stored frozen.
Measurement of vascular tone
Male Sprague-Dawley rats were killed by decapitation. The thoracic aorta was rapidly removed, cleaned from the adjacent tissue and cut into four rings of approximately 4 mm in length. Two metal hooks were carefully passed through the lumen of each ring and then mounted in 25-ml organ baths containing Kreb’s solution under 2 g of tension. Kreb’s solution had the following composition (1 mmol/liter): NaCl, 118; KCl, 4.75; CaCl2, 2.54; MgSO4·7H2O, 1.2; KH2PO4, 1.19; NaHCO3, 23; Dextrose, 11; the pH of the solution was 7.35∼7.45. Rings were equilibrated for 90 min with solution changes every 15 min. The bathing solution was kept at 37°C and was continuously aerated with a mixture of 5% CO2and 95% O2.
After equilibrium was established, l-phenylephrine, PE (0.3 μmol/liter) was added to the organ bath and maximal contraction developed. Increasing concentrations of either acetylcholine (ACH), pravastatin (PRA) or simvastatin (SIM, open ring) from 1 nmol/liter to 10 μmol/liter, were added to the organ baths every 10 min and the degree of relaxation was monitored. Cumulative dose response curves (DRC) were constructed to ACH, PRA and SIM control over a period of 1 to 1.5 h. The bathing solution was drained and replaced with fresh solution. The rings were allowed to return to the baseline and another DRC was built using the same process as described above. When SIM in its native closed-ring form was examined (1 to 10 μmol/liter), control responses to DMSO (0.001 to 0.00001%) were obtained. All responses were expressed as a percentage of the maximal relaxation. In other experiments, the endothelium of the vascular rings were removed by gently rolling of the luminal surface of the rings over a small metal rod after its insertion into the vascular lumen.
Measurement of nitric oxide production by bovine aortic endothelial cells
NO electrode method
Nitric oxide production was measured with a NO meter (MARK II ISO-NO, World Precision Instruments, Sarasota, Florida) connected to a polargraphic NO electrode as previously described (16). Bovine aortic endothelial cells (BAECs) were obtained from Cell Systems (Kirkland, Washington) and expanded in 150-mm plates (Corning) to passages 4-6. The growth medium, medium-199, was supplemented with penicillin G (100 U ml−1), streptomycin (100 mg ml−1), glutamine (100 mg ml−1), thymidine (100 mg ml−1) and 10% fetal calf serum (Gibco). For experiments, BAECs were grown to confluence on 24-well plates. Before NO measurement, the cells were washed twice with phosphate buffered saline (PBS) and then bathed in fresh medium-199. The NO sensor probe was next inserted vertically into the wells such that the tip of the electrode was submerged 2 mm under the surface of the medium. The electrode was routinely calibrated with graded concentrations of S-Nitroso-N-acetyl-DL-penicillamine (SNAP), which produces [NO] from 2 to 250 nmol/liter to obtain a standard curve.
The wells containing the confluent BAECs were randomly divided into three treatment groups: 1) ACH, PRA or SIM (0.1 to 10 μmol/liter); 2) ACH, PRA or SIM (1 μmol/liter) after pretreatment with the NOS inhibitor l-NAME (1 mmol/liter, 30 min); 3) SIM (1 to 10 μmol/liter) in the closed lactone ring form. The peak reading of the meter represented the amount of NO measured. The reaction was initiated when agents in volumes of 10 μl were added to achieve the desired concentration. Basal levels of NO varied between 46 and 67 nmol/liter (mean: 57 nmol/liter). Values for basal NO were subtracted from any drug-induced responses to determine NO production resulting solely from the action of each drug.
Methemoglobin method
The effects of acetylcholine and pravastatin on NO production in BAECs were determined using a highly sensitive photometric assay for conversion of oxyhemoglobin to methemoglobin. Nitric oxide oxidizes oxyhemoglobin (HbO2) to methemoglobin (metHb) in the following reaction: HbO2+ NO → metHb + NO−3. Therefore, the amount of NO produced by endothelial cells can be quantified by measuring the change in absorbency as HbO2oxidizes to metHb. Oxyhemoglobin has an absorbency peak at 415 nm, whereas metHb has a 406-nm absorbency peak. By subtracting the absorbency of metHb from HbO2, the concentration of NO can be assessed. Our general method was patterned after that of Feelisch and Kelm (17)(see Fig. 1).
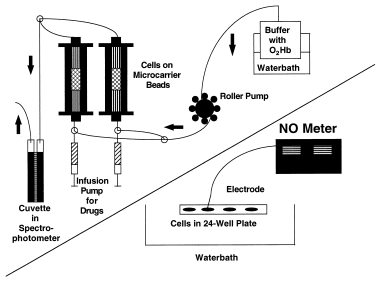
1. Download : Download high-res image (106KB)
2. Download : Download full-size image
Figure 1. Diagram illustrating methods used to measure NO production. The upper left half of the figure illustrates the methemoglobin method for measuring NO production in endothelial cells. The bottom half of the figure illustrates the NO electrode method for measuring NO production in endothelial cells.
For these experiments BAECs were prepared on microcarrier beads (Cytodex #3, 10−7cells/0.5 g beads). After plating, cells, beads and medium were transferred to a spinner flask (Wheaton), which was incubated at 37°C with 95% O2and 5% CO2. The culture was left undisturbed for 29 min and spun (20 rpm) in the same environment for 1 min. These resting and spinning cycles were then repeated for 4 h. This sitting cycle allowed for cell adherence to the beads while the spinning created an even distribution of cells and beads. At the end of this attachment phase, the spinner flask was left on the stirrer at slow speed for 2 to 3 days to allow for uniform cellular coating of beads.
For experiments, beads with cells were rinsed twice with PBS and then suspended in a Hepes-buffered Krebs-Ringer solution containing all necessary co-factors. To prevent a reaction between NO and superoxide (O2·−), superoxide dismutase (200 U/ml) was added to the buffer. Catalase (100 U/ml) was added to decompose hydrogen peroxidase, keeping the hemoglobin active. Next, 2 ml of beads with cells were placed into a water-jacketed chromatography column (Pharmacia Biotech) and superfused at 2 ml/min with 3 μmol/liter oxyhemoglobin. The perfusate was then directed into a flow-through cuvette in a dual-wavelength spectrophotometer, and absorbency was measured to determine the basal and stimulated NO release. A parallel column circuit was filled with only beads (no cells) to determine basal and spontaneous release of NO in the system.
Experimental stimulation was carried out by 3-min infusion periods of ACH or PRA added to buffer perfusion using a microsyringe pump at a rate of 45 μl/min to yield a final concentration of 1 and 10 μmol/liter for both ACH and PRA in the buffer. Vehicle (buffer without agent) did not cause a change in absorbency when infused into the cell/bead column. The effects of buffer containing l-NAME (1 mmol/liter) in blocking the actions of these drug agents and then a buffer without l-NAME but with excess l-ARG (1 mmol/liter) in reversing any l-NAME effect were also examined. Each drug agent concentration was given twice for each of the three buffer systems; a period of 10 min was allowed between infusion of agents. Our data demonstrate that this cell perfusion and monitoring system remains stable for at least 4 to 6 h. At the end of each experiment, cell viability was checked using trypan blue exclusion.
For analysis, we determined the area under the curve for the change in absorbency response/min caused by each agent above baseline levels. Production of MetHb was calculated using an extinction coefficient of 39 mmol/liter−1/cm−1. During the 3-min infusion of agents, absorbency increased rapidly. Changes in absorbency to these agents usually persist from 2 to 8 min depending on the size of the response before returning to baseline levels. We assume a one-to-one correspondence for NO and metHb production, the known stoichiometric balance for this reaction. We also determined levels in basal NO production during perfusion with each of the buffer systems. Basal levels of NO varied between 49 and 174 nmole/min (mean: 105 nmole/min). Basal NO values were subtracted from the drug-induced responses to determine NO production resulting solely from the drug action.
Data analysis
The data are expressed as mean values ± SEM. Data in Figure 2, Figure 3, Figure 4are absolute values for experimental determinations. Data in Figure 3, Figure 4are expressed as a percentage of control responses in Figure 3, Figure 4, respectively. One-way analysis of variance was used to determine whether differences exist among treatment groups. The Newman-Kuels post hoc test was also employed. A probability value of less than 0.05 was considered statistically significant.
1. Download : Download high-res image (110KB)
2. Download : Download full-size image
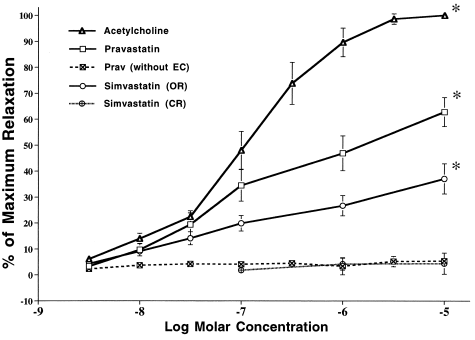
Figure 2. Vasorelaxation response of preconstricted aortic rings to increasing concentrations of ACH, PRA and SIM. Responses to ACH and PRA were obliterated following removal of the endothelial cells. Responses to SIM were seen only with the open lactone ring form (OR) of the compound (responses to SIM in the closed lactone ring preparation (CR) were no different from those produced by the DMSO vehicle used to solubilize the compound). (∗) Indicates difference from responses to all other agents or pretreatment. p< 0.05.
1. Download : Download high-res image (388KB)
2. Download : Download full-size image
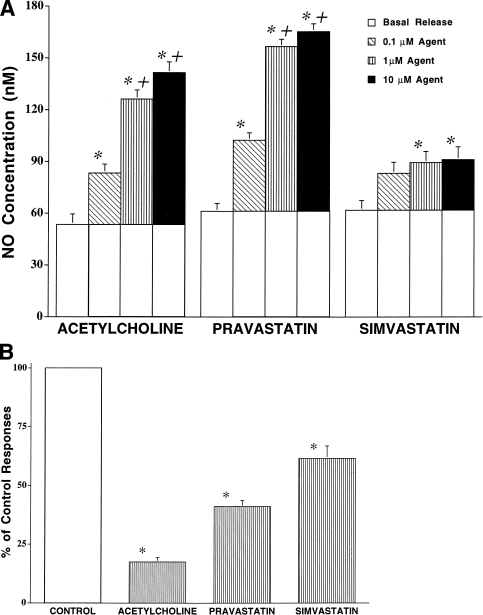
Figure 3. (A) NO electrode assay of effects of ACH, PRA and SIM on NO release by cultured BAECs. Confluent cultures were prepared in 24-well plates and treated with 0.1 to 10 μmol/liter concentrations of ACH, PRA and SIM (open lactone ring preparation). Open barsindicate basal NO release. Shaded barsindicate responses to agents above basal production. (∗) Indicates difference from basal release (control); (†) indicates difference from response at 0.1 μmol/liter, p < 0.05. (B) NO electrode assay of l-NAME effects on BAEC responses to 1 μmol/liter of ACH, PRA and SIM (open ring preparation only) as a percent of control responses to these agents (A). (∗) Indicates difference from control responses for that agent and dose. p < 0.05.
Results
Vascular tone
To test whether PRA could have an effect on endothelial cell function independent of its lipid lowering actions, we first compared the PRA and SIM vasorelaxing actions with those of ACH using a model that has been well established for demonstrating agents’ effects on endothelium-dependent vasorelaxation. Acetylcholine, PRA and SIM all produced relaxation of isolated aortic rings but exhibited different potencies (Fig. 2). The maximum vasorelaxations to ACH, PRA and SIM occurred at 10 μmol/liter and were 100 ± 0%, 62.8 ± 5.6% and 37.1 ± 5.8%, respectively. Relaxation responses to PRA and SIM occurred with the same latency, ∼8 min. Responses to ACH occurred more rapidly, 1.5 to 2 min. Removal of the endothelium from the aorta completely prevented relaxation responses to both PRA and ACH. Simvastatin in the close lactone ring form did not affect vascular tone.
Nitric oxide production
To determine and directly compare the actions of ACH, PRA and SIM in stimulating NOS activity and NO release, production of NO was assayed in cultured BAECs using the NO-sensitive electrometer. To further confirm the effects of PRA on endothelial cell NOS activity and NO formation and to test the reversibility of the l-NAME effects in blocking BAEC responses to PRA, we used the methemoglobin (MetHg) method for assaying NO release.
NO electrode method
Acetylcholine, PRA and SIM (0.1 to 10 μmol/liter) stimulated NOS activity and NO release (Figs. 3, A and B). Responses were complete within 10 min. Both ACH and PRA at 10 μmol/liter stimulated release of NO to similar peak responses above basal levels—88 and 103 nmol/liter, respectively—in cells with 50 μmol/liter l-ARG in media. Lower concentrations of these agents produced smaller increments in NO production. Moreover, SIM stimulated NO release, but the effects were weak with a peak response of 34 nmol/liter. The effects of 0.1 and 1 μmol/liter SIM were similar to those of 10 μmol/liter, suggesting that the peak effect was reached at the lower concentration. The native, closed-ring form of SIM produced similar NO responses above basal levels of 24.4 ± 1.7, 26.7 ± 1.4 and 26.0 ± 1.3 nmol/liter at concentrations of 0.1, 1 and 10 μmol/liter, respectively.
Pretreatment of cells with l-NAME (1 mmol/liter) blunted the NO responses to 10 μmol/liter ACH, PRA and SIM (open-ring form) to 18, 41 and 70% of control values, respectively (Fig. 3B).
MetHb method
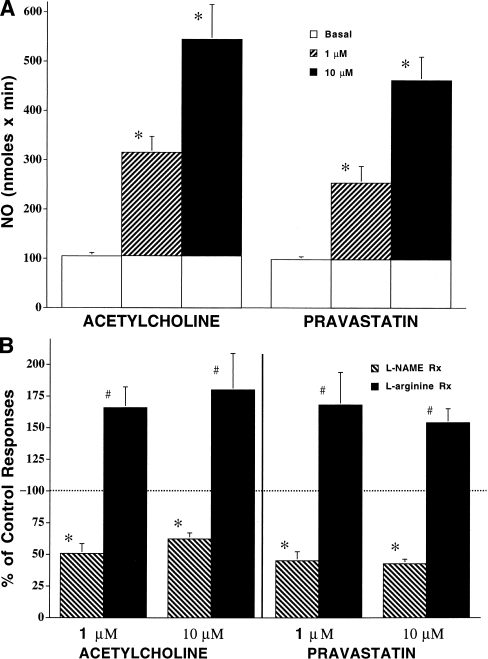
These experiments confirmed a concentration-dependent effect of both ACH and PRA on BAEC production of NO (Fig. 4A and B). The BAEC treated with 1 and 10 μmol/liter ACH in the presence of 10 μmol/liter l-ARG) increased their NO release from 227 to 521 nmol/min. Cells treated with 1 and 10 μmol/liter PRA increased their NO release from 185 to 413 nmole/min. Nitric oxide production with both agents was seen within the first 30 s. The l-NAME (1 mmol/liter) reduced ACH responses to 51% and 60% of control values, whereas responses to PRA were reduced to 45% and 43% of control values. Addition of extra l-ARG (1 mmol/liter) to the media reversed the action of l-NAME and potentiated NO production in response to ACH and PRA to 166% and 180% versus 168% and 154% of control values, respectively.
1. Download : Download high-res image (324KB)
2. Download : Download full-size image
Figure 4. (A) Methemoglobin assay of ACH and PRA effects on NO release from cultured bovine aortic endothelial cells. BAECs (2 ml) grown on microcarrier beads were perfused with oxyhemoglobin (3 μmol/liter) buffer containing 50 μmol/liter l-arginine at 2 ml/min and then stimulated with acetylcholine and pravastatin (1 and 10 μmol/liter, 3 min, n = 7). Open barsindicate basal NO release. Shaded barsindicate responses to agents above basal production. (∗) Indicates difference from basal release (control); (†) indicates difference from response at 0.1 μmol/liter p < 0.05. (B) Methemoglobin of BAEC responses to ACH and PRA in the presence of l-NAME and excess l-arginine as percent of control responses to these agents. Bars depict NO formation using the buffer above supplemented with l-NAME (1 μmol/liter, dark bar) or l-arginine (1 μmol/liter, light bar) stimulated with acetylcholine and pravastatin (1 and 10 μmol/liter, 3 min, n = 7). (∗) indicates difference from control responses for that agent and dose; (#) indicates difference from responses with l-NAME and control response for that agent and dose. p < 0.05.
Discussion
Summary
Paravastatin (PRA) stimulated vasorelaxation responses in aortic rings. Removal of the endothelium from aorta strips obliterated vasorelaxations produced, indicating the endothelial cell-dependency of the response. Also, PRA stimulated increases in NO production by cultured endothelial cells similar to the effects seen with ACH. This was demonstrated by directly measuring NO production with a NO-sensitive electrode and confirmed by measuring NO production using a highly sensitive MetHb assay. The NO production was inhibited by the NOS inhibitor l-NAME and reversible upon the addition of excess l-arginine to the medium, demonstrating specificity of the effect. Simvastatin was only 25% to 30% as effective as PRA in inducing vasorelaxation and NO release. No vasorelaxation responses were observed with the closed lactone ring form of SIM. In the open-ring form, SIM was slightly more effective in stimulating NO production than it was in the closed-ring form.
Rationale
This study was designed to determine the ability of PRA and SIM to stimulate NO production by vascular endothelial cells independent of their effects on hepatic cholesterol metabolism and to correlate NO production with vasorelaxation responses. It has been suggested that increased plasma LDL inhibits active transport of l-ARG by endothelial cells (18), uncoupling the l-ARG:eNOS pathway and leading to superoxide anion production (19). Because both superoxide anion and its reaction product with NO, peroxynitrite (OONO·−), produce tissue injury (20), it is possible that an indirect action of PRA, and other 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, may be to normalize endothelial function by protecting the active arginine transporter from injury by LDL, thereby preventing the formation of O2·−and OONO·−19, 20. These adverse effects of elevated LDL in increasing superoxide anion levels can be inhibited or reversed by l-ARG treatment 19, 21.
In our study the use of isolated endothelial cells maintained in culture precluded the influence of PRA’s actions in lowering hepatic production of LDL. Thus, our data suggest that in addition to the indirect effects of PRA in preserving eNOS activity by preventing endothelial cell injury caused by excess plasma LDL, PRA may have a direct action on endothelial cells. We postulate a direct action of stimulating endothelial cell NO production independent of effects on cholesterol metabolism for several reasons.
First, the fact that PRA stimulates NO production by cultured endothelial cells clearly rules out the contribution of its well-established actions in reducing plasma LDL levels. Moreover, the rapid time course of the endothelial cell response to PRA similar to that seen with ACH, a known eNOS agonist, makes it very unlikely that the increase in NO production could be a result of PRA effects on cholesterol metabolism in the cultured cells. Endothelial cell nitric oxide synthase activity is known to decrease with elevation of membrane cholesterol levels (19)and HMG-CoA reductase inhibitors have been shown to inhibit cholesterol synthesis in cultured endothelial cells 22, 23. However, it is very unlikely that significant alterations in endothelial cell plasma membrane cholesterol could occur within minutes of PRA addition to the cultures. For this reason, it is unlikely that PRA-induced reductions in membrane cholesterol levels could contribute to its acute actions in stimulating NO release. Furthermore, studies of PRA in cultured endothelial cells have shown that cellular uptake of this polar compound is limited and that its ability to inhibit LDL synthesis is relatively limited compared with other “vastatin” agents (23).
Finally, the fact that similar PRA effects on NO production were observed in three different assay conditions (one done in presence of serum-containing medium and two done in serum-free buffer) indicates that the presence or absence of LDL in the culture environment did not affect the results. This strongly supports a mechanism independent of the PRA effects on cholesterol metabolism.
Recently, SIM and lovastatin have been shown to upregulate eNOS gene expression over a period of hours (24). However, increases in eNOS expression could not contribute to the acute changes in NO production that occurred within minutes in our study. Therefore, our results suggest an additional, independent mechanism for the beneficial effects of PRA in treatment of cardiovascular disease processes.
Clinical implications
Production of endothelium-derived nitric oxide may explain the previously reported beneficial effects of PRA in inhibiting platelet thrombus formation (3)and in reducing myocardial infarction (MI) and stroke beyond the levels that could be accounted for by its effect in reducing LDL cholesterol levels (1). Promotion of NO production may also account for the reductions seen in recurrent MI, stroke, need for bypass surgery, and coronary angioplasty in patients without hypercholesterolemia (2). This action would also explain the vasodilator actions of PRA recently reported (25). Furthermore, our data from cultured cells and from data in humans (26)suggest that this mechanism may be amplified clinically by administering PRA in arginine-based delivery systems.
The results of our study and a recent study of hypertension (27)underscore the importance of avoiding the use of surrogate end points (28)such as reductions in LDL or blood pressure as the principal criteria for choosing drugs in the treatment of cardiovascular diseases. Reinforcing this point is the recent finding that another statin, atoravastatin, has an auxiliary action of increasing fibrinogen (29). This may offset its beneficial effect on LDL lowering. The effects of atoravastatin on primary end points remain to be determined.
Differences in the efficacy of l-NAME in inhibiting NO responses to PRA and SIM are not readily explained and remain under investigation. Differences in vasorelaxation to PRA and SIM can be explained by the differences in degree of NO production by these two agents. Although ACH and PRA were about equally effective in producing NO, ACH was a more potent vasorelaxant than was PRA. We believe this is because of ACH’s ability to produce vasorelaxation in rat aorta through mechanisms other than NO release—that is, endothelium-derived hyperpolarization factor (30). Characteristics of the vasorelaxation to PRA are comparable to those we have previously observed with substance P (31).
It should be noted that vasorelaxation responses to SIM were seen only with the open lactone ring preparation and that SIM in the open-ring form was only 25% to 30% as effective as PRA in producing NO. Coupled with the fact that only 5% of SIM circulates in the open-ring form (6), this could explain the failure of SIM to inhibit platelet aggregation as has been found with PRA (3). It would also explain the findings that clinical benefits of SIM appear more directly related to the magnitude of the change in LDL levels than those seen with PRA 32, 33, 34.
eNOS activation
An auxiliary mechanism of PRA as an eNOS agonist apart from its principal therapeutic mechanism is not unique to PRA. In addition to those agents previously discussed 8, 9, 10, 11, 12, 13, 14, 15, our data in isolated rat aortic rings and bovine aortic endothelial cells as well as in humans suggest that nitroglycerin (GTN) elevates cGMP levels and NO production by an l-arginine-dependent and l-NAME-sensitive mechanism in addition to its known action of elevating cGMP with NO derived from thiol-dependent enzymatic denitration of GTN 35, 36, 37.
The mechanism(s) by which PRA stimulates NO production in the endothelial cells is not known. It may act upon a specific cell membrane receptor to allow entry of extracellular calcium activating eNOS or it might act on membrane components rather nonspecifically to activate eNOS or enhance exposure of the enzyme to l-ARG. Whatever the process, our data demonstrate that PRA causes an immediate release of NO from these cells.
Conclusions
Paravastatin acutely activates eNOS to a similar extent as ACH. Simvastatin also activates eNOS but is much less effective. Vasorelaxation responses to PRA were comparably greater than those seen with SIM. Previously observed clinical benefits unrelated to LDL reduction and differences in platelet inhibition between PRA and SIM may be the result of the greater effectiveness of PRA as a NOS agonist.