

 2021-09-02
2021-09-02
Abstract
ABSTRACT. Limited data suggest that HMG-CoA reductase inhibitors (statins) may slow loss of renal function in individuals with chronic renal insufficiency. This study was conducted to determine whether pravastatin reduced rates of loss of renal function in people with moderate chronic renal insufficiency. This was a post hoc subgroup analysis of a randomized double-blind placebo controlled trial. Data were analyzed from the CARE study (a randomized trial of pravastatin versus placebo in 4159 participants with previous myocardial infarction and total plasma cholesterol < 240 mg/dl). Participants with estimated GFR (MDRD-GFR) < 60 ml/min per 1.73 m2 body surface area at baseline were considered to have moderate chronic renal insufficiency. Multivariate regression was used to calculate rates of decline in MDRD-GFR for individuals receiving pravastatin and placebo, controlling for prospectively determined covariates that might influence rates of renal function loss. Change in renal function could be calculated in 3384 individuals, of whom 690 (20.4%) had MDRD-GFR < 60 ml/min per 1.73 m2 and were eligible for inclusion. Among all individuals with MDRD-GFR < 60 ml/min per 1.73 m2), the MDRD-GFR decline in the pravastatin group was not significantly different from that in the placebo group (0.1 ml/min per 1.73 m2/yr slower; 95% CI, −0.2 to 0.4; P = 0.49). However, there was a significant stepwise inverse relation between MDRD-GFR before treatment and slowing of renal function loss with pravastatin use, with more benefit in those with lower MDRD-GFR at baseline (P = 0.04). Rate of change in MDRD-GFR in the pravastatin group was 0.6 ml/min per 1.73 m2/yr slower than placebo (95% CI, −0.1 to 1.2; P = 0.07) in those with MDRD-GFR < 50 ml/min, and 2.5 ml/min per 1.73 m2/yr slower (95% CI, 1.4 to 3.6 slower; P = 0.0001) in those with MDRD-GFR < 40 ml/min per 1.73 m2/yr. Pravastatin also reduced rates of renal loss to a greater extent in participants with than without proteinuria at baseline (P = 0.006). It is concluded that pravastatin may slow renal function loss in individuals with moderate to severe kidney disease, especially those with proteinuria. These findings require confirmation by a large randomized trial conducted specifically in people with chronic renal insufficiency. E-mail: mtonelli@ualberta.ca
Chronic renal insufficiency is a common condition that is associated with variable rates of progression to end-stage renal disease (1). Interventions known to delay or prevent progression include tight control of BP (2) and blood glucose (3) and the use of angiotensin-converting enzyme inhibitors (4,5⇓) and angiotensin II receptor antagonists (6,7⇓). However, even with optimal medical management in the context of clinical trials, patients with renal insufficiency are at risk for progressive loss of kidney function (6).
Hyperlipidemia has been hypothesized to play an important role in the progression of renal insufficiency, either through toxic effects of lipids on mesangial cells or by promoting intrarenal atherosclerosis (8–10⇓⇓). In addition, there is emerging evidence that chronic renal insufficiency may be associated with chronic inflammation (11–13⇓⇓), although it is controversial whether inflammation mediates progressive renal disease. Drugs that inhibit HMG-CoA reductase (statins) reduce serum cholesterol directly and may have additional antiinflammatory effects (14). Although data from several small studies suggest that that statins might slow the rate of renal function loss (reviewed in reference 15), results from large randomized trials are unavailable.
The CARE study was a randomized trial of pravastatin versus placebo in 4159 individuals with hyperlipidemia and a history of myocardial infarction (16). Participants were prospectively followed for new coronary and cardiovascular events for a median of 5 yr. We analyzed data from the subset of CARE participants with at least moderate chronic renal insufficiency, as defined by estimated GFR < 60 ml/min per 1.73 m2 body surface area (BSA). GFR was estimated using an equation from the Modification of Diet in Renal Disease study (MDRD-GFR) (17). We tested the hypothesis that pravastatin would slow the rate of decline in renal function.
Materials and Methods
Study Design and Patients
The design of the CARE trial has been described in detail elsewhere (18). Briefly, men and postmenopausal women were eligible if they had had an acute myocardial infarction between 3 and 20 mo before randomization, were 21 to 75 yr of age, and had plasma total cholesterol levels of less than 240 mg/dl (6.2 mmol/L), LDL cholesterol levels of 115 to 174 mg/dl (3.0 to 4.5 mmol/L), fasting triglyceride levels of less than 350 mg/dl (4.0 mmol/L), fasting glucose levels of no more than 220 mg/dl (12.2 mmol/L), left ventricular ejection fractions of no less than 25%, and no symptomatic congestive heart failure. Patients with proteinuria ≥ 2+ on routine dipstick or serum creatinine levels > 1.5 times the upper limit of normal for the central study laboratory were excluded from the CARE study.
After stratification according to clinical center, eligible participants were assigned by computer-generated random order to receive either 40 mg of pravastatin (Pravachol, Bristol Myers Squibb) once daily, or placebo. Treatment allocation was concealed using a centrally maintained code. Serum creatinine measurements using the alkaline picrate method of Jaffe were made annually by individuals at a central study laboratory who were blinded to treatment assignment. During the first 26 mo of the CARE study, measurements were made using the Olympus analyzer (Olympus, Melville, NY), and the AXON analyzer (Technicon, Tarrytown, NY) was used after this time. An extensive evaluation of the comparability of the methods was made, and the CARE dataset was corrected for the systematic difference between assays. Quality assurance was maintained through use of standard Westgard quality control multi-rules (19), and external proficiency was provided through the Interlaboratory Survey Program of the American College of Pathologists (Northfield, IL). On the basis of monthly averages of control materials analyzed several times daily, the percent coefficient of variation for creatinine measurement ranged from 0.8% at 9.0 mg/dl to 1.8% at 1.4 mg/dl. Internal and external quality assurance showed the measurement of creatinine to be stable and acceptably calibrated throughout the study.
Previous work suggests that treatment with pravastatin does not interfere with serum creatinine measurements (20) and that pravastatin pharmacokinetics are not affected by renal insufficiency (21). Inspection of the data set and comparison of changes in renal function during the first year of follow-up between treatment groups confirmed the absence of an acute effect on serum creatinine in association with pravastatin use.
Definitions of Renal Insufficiency
The primary index of renal function was MDRD-GFR, as calculated by the following equation :![]()
where PCr is plasma creatinine in mg/dl. This formula has been shown to agree closely with iothalamate measurements of GFR (17). Participants with MDRD-GFR < 60 ml/min per 1.73 m2 BSA at baseline were considered to have moderate chronic renal insufficiency, in agreement with recent guidelines (17).
The primary end point was the rate of change in MDRD-GFR (ml/min per 1.73 m2 BSA per yr). Secondary end points included the rate of change in estimated creatinine clearance as calculated by the Cockcroft-Gault equation (22) (ml/min per yr) and the proportion of individuals with sustained increases in serum creatinine of ≥0.3, ≥0.4, or ≥0.5 mg/dl during follow-up, using time-to-event analyses. Slopes of the rate of loss in renal function during the study were calculated for MDRD-GFR and creatinine clearance.
We included only those participants from the original CARE study for whom the data needed to calculate these estimates of renal function were available on four or more occasions, representing at least 3 yr of follow-up. Proteinuria was defined as trace proteinuria or greater on routine urinalysis at baseline. We repeated analyses in subsets with more severe renal impairment (MDRD-GFR < 50 and < 40 ml/min per 1.73 m2), and using creatinine clearance to estimate renal function.
Statistical Analyses
We analyzed participants in the groups to which they were randomized, and P values are two-sided. Multivariate linear regression analyses were performed to determine the effect of treatment assignment on rates of renal function loss. Age, race, gender, baseline renal function, diabetic status, use of angiotensin-converting enzyme inhibitors, systolic and diastolic BP, baseline HDL, LDL, and total cholesterol, baseline triglycerides, and pravastatin use were prospectively selected as independent variables, and the rate of renal function loss was the dependent variable. Values are reported as mean ± SD or percentages; 95% CI are provided where appropriate. The rate of change in renal function was computed from a two-stage regression procedure. In the first stage, each individual who had follow-up data that permitted estimation of MDRD-GFR for at least 3 yr had annual MDRD-GFR computed and regressed onto time. This produced for each individual the slope of the MDRD-GFR and the variance of that slope. These slopes were then used as the dependent variable in a weighted regression model, with the inverse variance of the slopes used as the weights and the same group of independent variables. Tests for interaction between the effect of pravastatin use and other independent variables (proteinuria, baseline renal function) on the dependent variable were performed by inserting a cross-product term in the model. All analyses were performed with SAS version 8.2 software (Cary, NC).
Role of the Funding Source
The CARE trial and this substudy on renal insufficiency were investigator-initiated studies funded by Bristol Myers Squibb. The data were collected and analyzed by and are now maintained at the Coordinating Center, University of Texas School of Public Health. The authors had unlimited access to the data used in this analysis. The sponsor is entitled to comment on manuscripts before submission, and the authors may consider these comments, but the rights to publication reside contractually with the investigators. The sponsor maintained information on adverse events and other trial data, as required by federal regulation.
Results
Baseline Characteristics
Trial flow is shown in Figure 1. There were 3384 CARE participants for whom renal function could be calculated on at least four occasions and were thus eligible for this reanalysis. Of these, 703 (20.8%) had renal insufficiency as defined by MDRD-GFR < 60 ml/min per 1.73 m2 at baseline. In 13 (0.4%) participants, values for one or more covariates were missing, necessitating their exclusion. The demographic characteristics of the remaining 690 participants are shown in Table 1. Mean baseline MDRD-GFR was 52.9 ± 6.5 ml/min per 1.73 m2, and median duration of follow-up was 58.9 mo in the individuals with renal insufficiency. The use of angiotensin-converting enzyme inhibitors was similar in pravastatin and placebo groups at baseline and during follow-up. Detailed analyses evaluating the relationship between rate of renal function loss and length of follow-up time showed no evidence of informative censoring with respect to renal function (23). Even within analyses that stratified participants by renal function at baseline, there was no evidence of informative censoring within any stratum.
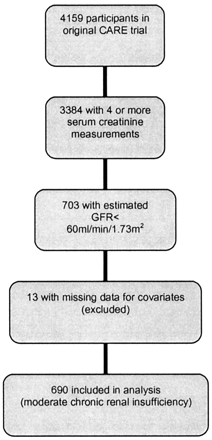
Figure 1. Flow of participants.
Table 1. Baseline characteristics of participants with moderate chronic renal insufficiency by treatment group
Effect of Pravastatin Use on Plasma Lipids
Among those with renal insufficiency, pravastatin lowered mean LDL cholesterol by 41 ± 26 mg/dl (1.0 ± 0.6 mmol/L) and mean total cholesterol by 41 ± 28 mg/dl (1.0 ± 0.6 mmol/L) compared with placebo. There were no significant changes in plasma triglycerides or HDL cholesterol during follow-up compared with placebo. Individuals with and without renal insufficiency had similar changes in plasma LDL cholesterol in response to pravastatin (data not shown).
Renal Function Loss in Individuals with Estimated GFR < 60 ml/min per 1.73 m2
Among the 690 individuals with moderate renal insufficiency at baseline, the mean rate of decline in MDRD-GFR was 0.6 ± 1.7 ml/min per 1.73 m2 per yr. Rates of MDRD-GFR decline were nonsignificantly higher in individuals with proteinuria at baseline (0.8 ± 1.9 ml/min per 1.73 m2 per yr) compared with those without (0.6 ± 1.6 ml/min per 1.73 m2 per yr; P = 0.17).
Overall, there was no difference in the rate of change in MDRD-GFR in those with renal insufficiency who received pravastatin, compared with controls (P = 0.49). Table 2 and Figure 2 show rates of renal loss in pravastatin and control groups among participants with moderate renal insufficiency.
Table 2. Effect of pravastatin on renal function loss in participants with and without moderate renal insufficiency at baselinea
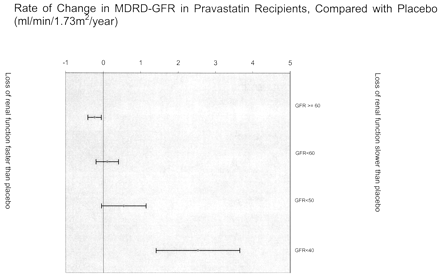
Figure 2. Adjusted effect of pravastatin on rate of renal function loss, stratified by baseline renal function. The figure shows annualized rate of change in renal function in pravastatin recipients compared with placebo. The effect of pravastatin on loss of renal function tended to be more beneficial in those with lower levels of renal function at baseline. Negative values reflect rates of change that were more rapid than placebo. Bars represent 95% CI. Proteinuria was defined by urinalysis that was positive for protein (trace or greater) at baseline. Models were adjusted for age, race, gender, baseline renal function, diabetic status, use of angiotensin-converting enzyme inhibitors, systolic and diastolic BP, baseline HDL, LDL, and total cholesterol, and baseline triglycerides, except for the MDRD-GFR < 40 subgroup, which was adjusted only for proteinuria, diabetic status, use of angiotensin-converting enzyme inhibitors, and pravastatin use due to the small number of participants.
Considering only those participants with moderate renal insufficiency at baseline, we observed a statistically significant interaction between baseline MDRD-GFR and the effect of pravastatin on renal function loss (P for interaction = 0.04). There were 176 individuals with MDRD-GFR < 50 ml/min per 1.73 m2, and 32 participants with MDRD-GFR < 40 ml/min per 1.73 m2 at baseline. Pravastatin treatment was associated with a statistically significant reduction in the rate of renal function loss among participants with MDRD-GFR < 40 ml/min per 1.73 m2 (Table 2, Figure 2).
The effect of pravastatin on renal function loss differed in individuals with and without proteinuria (P for interaction < 0.006). Pravastatin tended to reduce rates of MDRD-GFR decline to a greater extent in participants with proteinuria than those without (Table 3, Figure 3). For example, among individuals with MDRD-GFR < 40 ml/min per 1.73 m2 at baseline, pravastatin resulted in rates of decline that were 2.4 and 3.0 ml/min per 1.73 m2 per yr slower than placebo in nonproteinuric and proteinuric participants, respectively, although the 95% confidence intervals (CI) for the effect of pravastatin in these two groups overlapped.
Table 3. Adjusted effect of pravastatin on renal function loss in participants with moderate renal insufficiency at baseline, stratified by baseline proteinuriaa
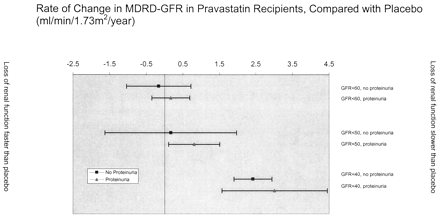
Figure 3. Adjusted effect of pravastatin on rate of renal function loss, stratified by baseline renal function and proteinuria. The figure shows annualized rate of change in renal function in pravastatin recipients compared with placebo. The effect of pravastatin on loss of renal function tended to be more beneficial in those with lower levels of renal function at baseline. For each level of renal function, proteinuria was more likely to be associated with benefit than lack of proteinuria. Negative values reflect rates of change that were more rapid than placebo. Bars represent 95% CI. Proteinuria was defined by urinalysis that was positive for protein (trace or greater) at baseline. Models were adjusted for age, race, gender, baseline renal function, diabetic status, use of angiotensin-converting enzyme inhibitors, systolic and diastolic BP, baseline HDL, LDL, and total cholesterol, and baseline triglycerides, except for the MDRD-GFR < 40 subgroup, which was adjusted only for diabetic status and pravastatin use due to the small number of participants.
Change in Renal Function Among Participants With MDRD-GFR ≥ 60 ml/min per 1.73 m2
Individuals with MDRD-GFR ≥ 60 ml/min per 1.73 m2 who received pravastatin had a small but statistically significant increase of 0.2 ml/min per 1.73 m2/yr in the rate of renal function loss compared with placebo (Table 2, Figure 2). The effect of pravastatin did not differ between groups with MDRD-GFR 60 to 75 and ≥75 ml/min per 1.73 m2, regardless of proteinuric status.
Alternate Definitions of Renal Function
Defining renal insufficiency and changes in renal function in terms of estimated creatinine clearance produced similar results to analyses in which MDRD-GFR was used. There were 513 participants who had creatinine clearance < 60 ml/min (15.1% of the cohort). Mean rate of decline in creatinine clearance in this subgroup was 1.8 ± 1.5 ml/min per yr. Participants receiving pravastatin had a statistically significant decrease in the adjusted rate of decline in creatinine clearance (Tables 2 and 3). Within this subgroup, proteinuria and lower creatinine clearance at baseline continued to influence the effect of pravastatin on rates of renal function loss (P for interaction = 0.007 and 0.0006, respectively).
Cox proportional hazards models were used to evaluate the relationship between treatment group and the likelihood of a sustained increase in serum creatinine during the study period in individuals with renal insufficiency at baseline. No differences were found in the risk of sustained increases of ≥0.3 mg/dl, ≥0.4 mg/dl, or ≥0.5 mg/dl between treatment groups.
Adverse Events in Renal Insufficiency
There were 120 participants with MDRD-GFR < 60 ml/min per 1.73 m2 who died during the study, 61 in the placebo group and 59 in the pravastatin group (P = 0.99). Among those with renal insufficiency, there were no significant differences in the number of participants in pravastatin and placebo arms who experienced asymptomatic elevations in serum creatine phosphokinase levels greater than three times the upper limit of normal (3 versus 2; P = 0.99) and no cases of rhabdomyolysis on pravastatin (versus 2 in control group; P = 0.24). The number of participants who developed depression (6 versus 13; P = 0.16), non-dermatologic malignancy (61 versus 75; P = 0.67), and skin cancer (25 versus 29; P = 0.25) were also similar between treatment groups.
Discussion
We found that pravastatin significantly reduced the rate of decline in renal function in individuals with more severe chronic renal insufficiency. Among individuals with MDRD-GFR ≥ 60 ml/min per 1.73 m2 at baseline, pravastatin use was associated with a clinically insignificant increase in the rate of subsequent renal loss. However, individuals with MDRD-GFR < 40 ml/min per 1.73 m2 who received pravastatin had rates of renal loss that were significantly less rapid than those who received placebo. In addition, individuals with proteinuria on dipstick and moderate renal insufficiency were more likely to experience a beneficial effect of pravastatin on renal function loss than those without proteinuria. The results were similar when estimated creatinine clearance rather than MDRD-GFR was used as the index of renal function.
Pravastatin reduced rates of decline in MDRD-GFR by 2.5 ml/min per yr (95% CI, 1.4 to 3.6) in participants with MDRD-GFR < 40 ml/min per 1.73 m2 at baseline. To put the magnitude of this effect into context, interventions of accepted value such as tight BP control and the use of angiotensin-converting enzyme inhibitors in patients with heavy proteinuria reduce rates of renal loss by 3.5 and 4.2 ml/min per yr, respectively (2,24,25⇓⇓). Our findings suggest that statins may also have a role in slowing rates of renal function loss in individuals with renal insufficiency.
Our findings are consistent with a recent meta-analysis of 13 controlled trials, which found that lipid-lowering therapy was associated with a protective effect on renal function loss (1.9 ml/min per yr; 95% CI, 0.3 to 3.4 ml/min per yr) (15). The majority of the 404 participants in the included trials had diabetic nephropathy or the nephrotic syndrome, in contrast to the CARE participants. Only 14% of those in CARE had diabetes mellitus and individuals with heavy proteinuria were specifically excluded. In addition, mean plasma total cholesterol concentrations were considerably higher in the meta-analysis than in CARE and not all patients received statins. However, the mean severity of renal impairment reported in the meta-analysis appears comparable to that of the individuals in the current study.
Members of the statin class have been shown to have beneficial effects on renal hemodynamics (26), endothelial function (27), monocyte recruitment, mesangial cell proliferation, and mesangial matrix accumulation (10), as well as antiinflammatory (14,28⇓) and immunomodulatory (29,30⇓) actions. In theory, any of these mechanisms might mediate the putative renoprotective effects of statins. Given that plasma levels of inflammatory biomarkers correlate with severity of renal insufficiency (11,31⇓) and development of microalbuminuria (32,33⇓), it is tempting to speculate that antiinflammatory activity is responsible. While recent work suggests that levels of C-reactive protein (a biochemical marker of inflammation) do not correlate with rates of renal function loss, the case-mix of underlying renal disease in that trial probably differed substantially from the current study (34). The mechanism for pravastatin’s effect on renal function loss in people with established coronary disease thus remains unknown. Finally, the immunomodulatory (35) and anti-proliferative effects may vary substantially from agent to agent; it is therefore possible that other statins might affect renal function to a lesser or greater extent than pravastatin.
Pravastatin use appeared to be safe in this population, as reflected by the low rates of adverse events. The explanation for the slightly more rapid renal loss in pravastatin recipients with higher levels of baseline renal function is unclear. Pravastatin does not appear to influence in vivo measurements of serum creatinine (20), nor is it known to affect creatinine metabolism, at least in the short term. Regardless, the magnitude of the increased rate of loss was small, and the likelihood of any clinical consequence is low.
Our study has limitations that should be considered. First, this was a secondary analysis, the limitations of which are well described. Second, unlike most previous work evaluating the impact of statins in kidney disease, renal function in the current analysis was estimated using the MDRD-GFR formula and the Cockcroft-Gault equation, which are less accurate than nuclear isotope estimates of GFR. However, their major sources of error are related to calibration and random variation (36). While calibration error might have resulted in misclassification of some subjects with respect to renal insufficiency, it should not produce differential rates of change between treatment groups. In addition, every attempt was made to minimize the influence of random error through careful laboratory methodology.
Finally, because of the inclusion criteria for the original CARE study, the number of participants with more severe renal insufficiency was small. Future trials that seek to overcome this limitation will face the challenge of selecting appropriate participants. For example, it seems unlikely that people with previous myocardial infarction could be prospectively studied, because statins improve clinical outcomes in this population and the magnitude of benefit appears similar in people with and without normal kidney function (37). However, the participants in this latter study had only mild to moderate renal insufficiency. Thus, future investigations might include participants with more severe kidney disease, without myocardial infarction, or both.
The cause of renal disease in the CARE participants is unknown, and all had coronary disease; therefore, the generalizability of these findings is uncertain. However, given the frequent coexistence of renal and cardiovascular disease, such individuals are common in the general population (38), making our findings particularly relevant. This analysis predominantly concerns individuals with moderate renal dysfunction, and only a single semiquantitative assessment of proteinuria was made, which may underestimate its prevalence. Despite these caveats, our data provide justification for the use of statins in moderate to severe advanced renal disease, because strategies that reduce rates of renal function loss are often highly cost effective (39,40⇓).
In summary, pravastatin significantly reduced rates of decline in renal function in individuals with MDRD-GFR < 40 ml/min per 1.73 m2 and in those with MDRD-GFR < 50 ml/min per 1.73 m2 in association with proteinuria. There was also a significant stepwise inverse relationship between baseline kidney function and the effect of pravastatin on renal function loss. Our data suggest that individuals with moderate to severe kidney disease may derive clinically relevant renal benefit from the use of pravastatin, especially those with proteinuria. These findings require confirmation by a large randomized trial conducted specifically in this patient population.