

 2021-09-02
2021-09-02
Abstract
Proteinuria is an important risk factor for cardiovascular and renal morbidity and mortality. The effects of 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitor (statin) therapy on proteinuria in normolipidemic patients with well-controlled hypertension have not been studied. A total of 63 normolipidemic (total cholesterol <240 mg/dL) and proteinuric (300 to 3000 mg/d) patients with well-controlled blood pressure (<140/90 mm Hg) were randomized to receive either placebo (n=32) or pravastatin (10 mg/d; n=31) after a 3-month placebo period. Pravastatin lowered proteinuria after 6 months by 54% (P<0.0001). Creatinine clearance was stable throughout the study in the 2 groups. Despite unchanged plasma endothelin-1 levels throughout the study, urinary excretion of the peptide was decreased and significantly correlated with improvement in urinary protein excretion in pravastatin-treated patients (r=0.64, P=0.001). The urinary excretion of retinol-binding protein decreased after pravastatin administration, probably reflecting an improvement in tubular function. In contrast, the urinary excretion of IgG did not change significantly throughout the study in either group. Multivariate analysis revealed that proteinuria was only significantly correlated with statin use (P<0.0001, R2= 0.66). Linear regression analysis in the statin-treated group did not show any correlation between changes in lipid profiles and proteinuria regression. Thus, in addition to their primary function of antilipidemia, the addition of pravastatin to treatment for well-controlled hypertension may have an additive effect on reducing proteinuria independent of hemodynamics and lipid-lowering effects, possibly through inhibiting renal endothelin-1 synthesis and improving tubular function.
Epidemiological studies have shown that urinary protein excretion is a strong and independent predictor of renal outcome.1 Hypertension has been shown to be an important cause of end-stage renal disease.2 Ruilope et al3 illustrated the existance of overt proteinuria in 17.5% of patients with chronic and well-controlled hypertension. Therefore, although tight blood pressure control is known to be a crucial factor in preventing progression of renal disease, other factors are undoubtedly involved. Recent studies showed that in comparisons with other antihypertensive drug classes, angiotensin-converting enzyme (ACE) inhibitors4 and angiotensin II receptor (AR) blockers5 slow the progression rate of renal disease in patients with proteinuria. However, although progression is slowed, it is not arrested, indicating the need for adjunctive therapy. Thus, it is of interest to identify drugs other than ACE inhibitors and AR blockers to reduce proteinuria.
The 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors (statins) have been shown to have a beneficial effect in inhibiting tissue inflammation in patients with normal or low cholesterol levels,6 implying that statin therapy has many effects independent of changes in plasma cholesterol concentrations. In animal models of renal disease, administration of lovastatin has reduced proteinuria and the degree of glomerulosclerosis.7 Proteins filtered through the glomerular capillary might have intrinsic toxicity on the proximal tubular cells and a contributory role to the progression of renal damage. Protein overload of proximal tubular cells may induce a dose-dependent increase in the synthesis and release of endothelin-1 (ET-1) in a rat model of proteinuric renal disease.8 Blocking ET-1 has been reported to diminish proteinuria.9 Statins have been shown to reduce the synthesis of ET-1 at transcriptional levels.10 Several studies investigated the effect of statins on proteinuria in humans. All these studies were performed in patients with either untreated hypertension or after antihypertensive agents were washed out. No studies have been reported on what adjunctive therapy should be administered for further reducing proteinuria if blood pressure has been well-controlled by drugs, including ACE inhibitors and AR blockers. It was unknown whether a ceiling of renoprotection, provided by ACE inhibitors and AR blockers, exists. Thus, we investigated (1) whether pravastatin administration was effective in reducing proteinuria when given to patients with well-controlled hypertension, (2) the add-on effect of pravastatin on reducing proteinuria in patients already treated with AR blockers, and (3) whether renoprotection is mediated by inhibiting renal ET-1 synthesis.
Methods
Patients
This study was conducted prospectively in a parallel, double-blind, randomized, and controlled manner in a single medical center. Eligible patients were required to have stable, well-controlled hypertension with a seated diastolic blood pressure of ≤90 mm Hg and systolic blood pressure of 140 mm Hg at a 3-month screening period. Cuff blood pressure was selected for blood pressure measurements to reflect standard office-based clinical practice. Proteinuria was defined as urinary protein excretion exceeding 300 mg/d in 2 consecutive 24-hour urine samples without evidence of urinary tract infection or overt heart failure.
Patients were excluded from the study if they had diabetes mellitus, secondary hypertension, renal disease (serum creatinine codncentration ≥1.5 mg/dL or 133 μmol/L), proteinuria (≥3 g/d), hyperlipidemia (plasma total cholesterol level ≥240 mg/dL), or treatment with corticosteroids or nonsteroidal anti-inflammatory drugs. Before they were enrolled in this study, no patients had ever received cholesterol-lowering agents. To evaluate the add-on effect of pravastatin, all antihypertensive agents were kept constant throughout the study. Because there was a high incidence of persistent cough with ACE inhibitors in Chinese patients,11 which could reduce drug compliance, the AR blocker losartan was the recommended agent. Apart from the antihypertensive drugs, none of the patients were taking any other drugs. The patients were not given specific risk factor modification instructions such as diet control or smoking cessation.
After a run-in period of 3 months during which the previously mentioned entry criteria were evaluated, a total of 66 consecutive proteinuric patients were randomized to receive 10 mg of pravastatin or placebo for 6 month between December 1999 and October 2001. Each patient received a randomized code number, according to which the study assistant supplied the study drug. Special drug packaging was used to maintain blindness of treatment. A sealed envelope, with information on the treatment allocated, was kept in the clinical file of each patient. The study was conducted in accordance with good clinical practices and local regulations. All patients were assessed at the outpatient clinic every 4 weeks. Tolerability was assessed by using spontaneously reported adverse events at each visit. For comparison of glomerular and tubular function, 10 healthy, normotensive and normolipidemic control subjects without proteinuria were selected and matched according to age, sex, height, and body weight.
Laboratory Tests
Proteinuria was measured in 24-hour urine samples by using the trichloroacetic acid technique.12 These urine samples were provided by patients who were carefully instructed in proper collection methods. To verify a correct urine sampling, 24-hour urinary sodium and chloride excretion were checked. Three patients (one in the placebo group and two in the pravastatin group) with sodium and chloride excretion <80 mmol/d and/or >130 mmol/d were excluded from the analysis. Protein intake was monitored by 24-hour urinary urea nitrogen excretion tests using the following formula13: Protein intake (g/d)=nitrogen content of urea and nonurea nitrogen (an estimated value of 31 mg/kg for nonurea nitrogen) ×6.25. Renal function was determined by measuring creatinine clearance using 24-hour urine volume and urine and plasma creatinine concentrations. All patients had creatinine clearance >50 mL/min per 1.73 m2. In patients with preserved renal function, creatinine clearance is a good index of glomerular filtration rate.14
Plasma and urinary samples for ET-1 measurements were collected and extracted as previously described.15 Samples were immediately centrifuged at 3000g for 10 minutes, and the plasma was stored at −70°C until further analysis. ET-1 was measured by immunoassay (R&D System Inc). The detection limit was 1 pg/mL for ET-1. There was <1% cross-reactivity with big ET-22 to 38. Intra-assay and interassay coefficients of variation were 4.5% and 6.6%, respectively. Results were expressed as pg/mL for plasma and ng/g of urinary creatinine for urine.
Urinary protein excretion is determined by glomerular permeability and tubular reabsorption. To assess the roles of glomerular and tubular functions in the reduction of urinary protein excretion, we measured the changes of urinary IgG as a glomerular marker and retinol binding protein as a tubular marker. Samples were immediately centrifuged at 3000g for 20 minutes. Urine samples for retinol binding protein were stored at a pH of 6 to 8 with 1 mmol/L NaOH at −70°C until further analysis to avoid degradation. The analysis was performed within 4 months, during which no loss of urinary retinol-binding protein was observed.16 Because 24-hour urine sample would entail samples remaining at 37°C for several hours, which has the potential for significant losses of retinol-binding protein in the bladder, 17 a daytime random sample was used instead. Polyclonal antibodies raised in rabbits against retinol-binding protein were obtained from ImmunDiagnostik AG Ltd. The detection limit was 8 μg/L. Intra-assay and interassay coefficients of variation were 7% and 8%, respectively. Correction for variations in urine flow rate can be made by expressing retinol-binding protein as a ratio to creatinine.
Plasma concentrations of total and high-density lipoprotein (HDL) cholesterol and triglycerides were measured by enzymatic methods as previously described.18 Low-density lipoprotein (LDL) cholesterol was calculated by the Friedewald equation. A 24-hour urine sample (protein excretion, electrolytes, creatinine, nitrogen, ET-1, IgG), random urine sample (retinol-binding protein), serum (electrolytes, creatinine, ET-1, and lipid profiles), and blood pressure were measured at the beginning and at the end of the study. Patients were required to sign an informed consent form before undergoing screening procedures.
Statistics
All data were analyzed by the SPSS statistical software. The continuous variables are expressed as mean±SD. Because many variables were not normally distributed, nonparameteric statistical tests were used throughout. The differences of continuous parameters between the 2 groups were compared by the Mann-Whitney U test, whereas the Wilcoxon matched-pairs test was used to compare baseline and 6-month data values. The differential effect of losartan treatment in patients treated with pravastatin compared with patients receiving placebo (no pravastatin) was assessed with the test for interaction in 2-way ANOVA. Proteinuria was analyzed after logarithmic transformation for its skewed distribution. For the categorical parameters, the differences were compared by the χ2 test and by Fisher’s exact test if the case number was <5. Relationships between the changes were studied by use of Spearman correlation and multiple linear regression analysis. P<0.05 was considered statistically significant.
Results
Of the 66 patients enrolled in this study, 3 patients had incomplete 24-hour urine collection assessed by urinary sodium and chloride measurement. Thus, there were a total of 63 evaluable patients (32 in the placebo group and 31 in the pravastatin group). Table 1 presents the baseline and demographic characteristics of patients in each group. Pravastatin was very well tolerated by all patients, and none had any significant subjective side effects. Patient compliance with the treatment was confirmed by the significant effects on blood lipids (Table 2). The number of antihypertensive agents was similar in the 2 groups, with a mean number of 2.9 in the placebo group and 3.1 in the pravastatin group. Twenty-one patients on pravastatin received an average of 60±28 mg/d losartan and 19 patients in the placebo group received 58±28 mg/d. There were no significant changes in hemodynamic parameters after administering pravastatin (data not shown). There was no difference at baseline or at 6 months in protein intake as calculated form urea excretion between the 2 groups (Table 2). We observed no clinically meaningful changes in the parameters indicative of renal function, such as serum creatinine and creatinine clearance, during the course of the study in the 2 groups.
Table 1. Baseline Characteristics of Patients | |||||||
Parameters | Pravastatin (n=31) | Placebo (n=32) | P | ||||
Values are mean±SD. | |||||||
Age | 50±9 | 47±8 | NS | ||||
Gender, M/F | 18/13 | 21/11 | NS | ||||
Blood pressure, mm Hg | |||||||
Systolic | 117±10 | 123±10 | NS | ||||
Diastolic | 72±5 | 74±5 | NS | ||||
Mean | 87±5 | 90±5 | NS | ||||
Pulse pressure | 45±11 | 49±12 | NS | ||||
Duration of hypertension, y | 12.6±5.8 | 12.8±5.8 | NS | ||||
Body mass index, kg/m2 | 24.7±1.1 | 24.5±1.2 | NS | ||||
Cigarette smoking, n (%) | 16 (52) | 20 (63) | NS | ||||
Antihypertensive agents, n (%) | |||||||
AR blockers | 21 (68) | 19 (59) | NS | ||||
β-Blocking agents | 20 (65) | 26 (81) | NS | ||||
Calcium blocker | 24 (77) | 19 (59) | NS | ||||
Diuretics | 16 (52) | 17 (53) | NS | ||||
Others | 15 (48) | 12 (38) | NS | ||||
Table 2. TABLE 2. Lipid Profiles, Dietary Protein Intake, Renal Function, and ET-1 at Baseline and After 6 Months of Therapy | |||||||
Parameters | Pravastatin (n=31) | Placebo (n=32) | |||||
Baseline | Follow-Up | Baseline | Follow-Up | ||||
Values are mean±SD. | |||||||
*P<0.05 compared with respective baseline data. | |||||||
Cholesterol, mg/dL | |||||||
Total | 210±23 | 181±20* | 205±23 | 200±24 | |||
HDL | 36±4 | 39±4* | 37±4 | 38±3 | |||
LDL | 125±23 | 102±18* | 123±25 | 116±29 | |||
Triglycerides, mg/dL | 241±42 | 200±20* | 226±52 | 230±51 | |||
Dietary protein intake, g/d | 72±14 | 70±9 | 71±12 | 71±11 | |||
Creatinine clearance, mL/min per 1.73 m2 | 85±16 | 93±19 | 90±19 | 97±19 | |||
Plasma samples | |||||||
Serum creatinine, mg/dL | 1.13±0.20 | 1.15±0.17 | 1.05±0.17 | 1.03±0.18 | |||
ET-1, pg/ml | 1.80±0.60 | 1.70±0.52 | 1.84±0.60 | 1.75±0.57 | |||
Urine samples | |||||||
Protein excretion, mg/d | 1234±490 | 560±274* | 1193±507 | 1096±456 | |||
ET-1, ng/g urinary creatinine | 28.3±4.6 | 24.1±4.3* | 29.6±5.4 | 27.8±5.0 | |||
IgG, mg/d | 21.5±5.7 | 19.1±5.5 | 21.3±6.3 | 19.9±5.3 | |||
Retinol-binding protein, μg/mmol | 277±69 | 145±53* | 304±79 | 279±68 | |||
Lipid Profiles
The levels of total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides at baseline and after 6 months are listed in Table 2. Compared with the placebo group, pravastatin administration caused significant reductions in plasma total and LDL cholesterol levels and an increase in HDL cholesterol by 13%, 17%, and 7% (all P<0.05), respectively.
Effect on Renal Glomerular and Tubular Markers
The baseline values and the effects of pravastatin on renal glomerular and tubular markers are shown in Table 2. The baseline urinary excretion of markers for glomerular size-selectivity (IgG) and for proximal tubular function (retinol-binding protein) were pathologically elevated in the 2 groups compared with normal controls (13±5 mg/d for IgG; 62±21 μg/mmol creatinine for retinol-binding protein); values were similar to those of healthy subjects in previous studies.19 The proximal marker retinol-binding protein was significantly reduced during pravastatin treatment compared with baseline, whereas IgG levels remained stable throughout the study.
Proteinuria and ET-1
Changes in urinary protein excretion are shown in Table 2. Urinary protein excretion did not differ between the 2 groups at baseline. In pravastatin-treated patients, proteinuria was significantly reduced by 54% (from 1234±490 to 560±274 mg/d; P<0.0001) compared with baseline values. Because there was a large SD of proteinuria relative to the mean, proteinuria amount was logarithmically transformed to a normally distributed variable. In the statistical analysis of the transformed values, proteinuria amount remained stable throughout the study in the placebo group, but it was significantly reduced in patients treated with pravastatin.
To assess whether pravastatin administration interacts with the use of losartan, we analyzed the data by 2-way ANOVA. After 6 months of follow-up, the amount of proteinuria in the pravastatin group was 653±197 mg/d without AR blockers and 515±297 mg/d with AR blockers, whereas in the placebo group, the amount was 960±324 mg/d and 1189±516 mg/d, respectively (Figure 1). Patients treated with pravastatin had, on average, 59% less progression if cotreated with losartan compared with no losartan cotreatment (P=0.008), whereas in the placebo group, no effect of losartan treatment was observed (Table 3). Pravastatin administration induced a further reduction of proteinuria in patients already treated with AR blockers (−752±399 versus −64±216 mg/d in the placebo group; P<0.0001) and in patients without AR blocker treatment (−511±195 versus −146±305 mg/d in the placebo group; P=0.003).
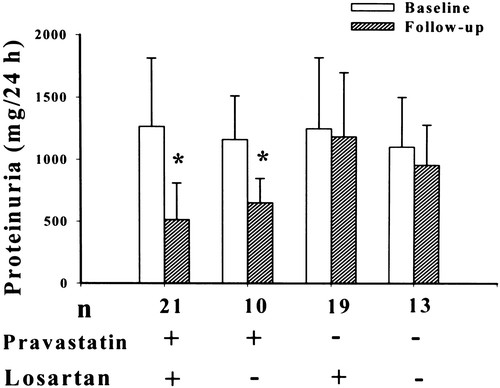
Figure 1. Twenty-four hour urine protein excretion classified by the use of pravastatin and losartan. *P<0.0001 compared with respective baseline.
Table 3. TABLE 3. Change of Lipid Levels and Renal Parameters During the Study With Regard to Losartan | ||||||
Parameters | Pravastatin | Placebo | ||||
With Losartan (n=21) | Without Losartan (n=10) | P | With Losartan (n=19) | Without Losartan (n=13) | P | |
Values are mean±SD and are expressed in absolute values or percent. | ||||||
ΔCholesterol, mg/dL | ||||||
Total | −35±19 | −18±23 | 0.04 | −5±21 | −5±26 | NS |
HDL | 2±2 | 3±3 | NS | 0±3 | 2±3 | NS |
LDL | −28±23 | −13±26 | NS | −4±24 | −11±32 | NS |
ΔTriglycerides, mg/dL | −41±58 | −38±41 | NS | −7±51 | 19±65 | NS |
ΔCreatinine clearance, mL/min per 1.73 m2 | 13±25 | −3±22 | NS | 9±15 | −3±19 | NS |
ΔPlasma ET-1, pg/mL | −0.02±0.49 | −0.27±0.68 | NS | −0.12±0.66 | −0.05±0.67 | NS |
ΔUrinary protein excretion, % | −59±17 | −43±9 | 0.008 | −3±17 | −9±27 | NS |
ΔUrinary ET-1, pg/mL | −4.7±1.8 | −3.2±1.5 | 0.03 | −2.2±6.5 | −1.2±4.1 | NS |
ΔUrinary IgG, mg/d | −3.0±7.1 | −1.1±9.3 | NS | −4.2±9.1 | 2.6±6.2 | 0.03 |
ΔUrinary retinol binding protein, μg/mmol | −148±68 | −97±36 | 0.03 | −51±93 | −2±71 | NS |
Baseline urinary ET-1 levels were similar in the patients with and without losartan, suggesting that losartan did not reduce the urinary ET-1 levels. Urinary ET-1 levels were significantly reduced in pravastatin-treated patients, whereas plasma ET-1 levels remained stable throughout the study.
Correlation
To identify determinants of a decrease in urinary protein excretion, multivariate analysis was performed (Table 4). Multivariate regression analysis with log proteinuria as the dependent variable and age, sex, changes in mean blood pressure, lipid profiles, and the presence or absence of pravastatin and losartan as independent variables was performed to investigate the effect of pravastatin and other risk factors on proteinuria. The use of statin was the only factor that was significantly related to proteinuria regression (P<0.0001, R2=0.66).
Table 4. Multivariate Analysis of Independent Factors for Regression of Proteinuria (model R2=0.66) | |||
Factor | Standardized β Coefficient | 95% CI | P |
CI indicates confidence interval. | |||
Treatment assignment (statin vs no) | 0.737 | −13.5–−7.6 | <0.0001 |
Losartan use, yes vs no | 0.091 | −4.3–1.6 | 0.36 |
Gender, male vs female | −0.028 | −0.2–1.1 | 0.76 |
Age (per additional y) | −0.110 | −4.3–1.1 | 0.24 |
Mean blood pressure (per additional mm Hg) | −0.183 | −0.01–0.51 | 0.07 |
Total cholesterol (per additional mg/dL) | −0.027 | −0.1–0.1 | 0.75 |
HDL cholesterol (per additional mg/dL) | 0.080 | −0.02–0.04 | 0.44 |
LDL cholesterol (per additional mg/dL) | −0.036 | −0.5–0.4 | 0.70 |
Triglycerides (per additional mg/dL) | −0.080 | −0.08–0.04 | 0.48 |
The linear regression models in the statin-treated group showed that changes in ET-1 correlated with urinary protein excretion (change in urinary ET-1 [%]=0.72×change in log proteinuria [%]+6.07; r=0.64, P=0.01; Figure 2). Changes in total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides were not a predictor of change in log urinary protein excretion (P=0.42, 0.47, 0.56, and 0.88, respectively). These data indicate the nonlipid effect of pravastatin on proteinuria. Additionally, no significant correlation was observed between changes in proteinuria and hemodynamics (systolic, diastolic, and mean blood pressure; data not shown).
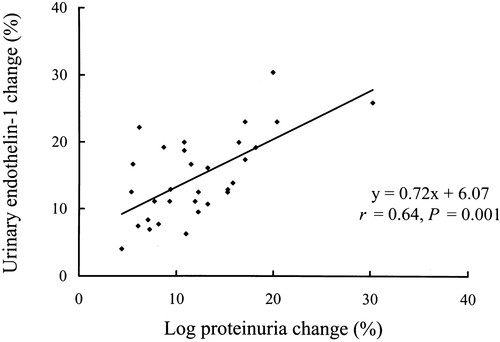
Figure 2. Correlation of the change (%) between log proteinuria and urinary ET-1 in the pravastatin-treated patients (r=0.64, P=0.001, n=31). A greater reduction of urinary ET-1 release was associated with a greater regression of proteinuria.
Discussion
Our results have demonstrated for the first time that pravastatin administration has additive beneficial effects on proteinuria independent of blood pressure reduction in patients with chronic and well-controlled hypertension by agents with or without AR blockers. The attenuation of proteinuria reflects an improvement in tubular function. Because of the significant correlation between proteinuria and urinary ET-1 levels, it is likely that the involved mechanism could be a direct inhibition of renal ET-1 synthesis by pravastatin. Thus, the results of the study demonstrate that the ceiling of renoprotection provided by AR blockade does not seem to be present for pravastatin administration.
Previous Studies
There were no previous studies concerning the effect of statins on proteinuria in well-controlled hypertensive patients without hyperlipidemia. There have been a number of small trials of the use of statins to treat hypercholesterolemic patients with or without diabetes mellitus. Pravastatin20 and simvastatin21 have been shown to have a beneficial effect on proteinuria in hypercholesterolemic type 2 diabetic patients. Rabelink et al22 showed that simvastatin substantially reduced albuminuria in hypercholesterolemic nondiabetic patients. In contrast, Deslypere et al23 reported that simvastatin induced proteinuria on one or more occasions in 10 of 120 hypercholesterolemic patients. The discrepancy could be explained by different patient population, implying different patterns of renal pathology. Caetano et al24 showed that hypertensive nephrosclerosis can be a definite cause of chronic renal failure and proteinuria. To avoid all possible confounders,25 only patients without hyperlipidemia and diabetes mellitus were included in this study. Besides, an influence of antihypertensive agents and protein intake, which are major determinants of proteinuria,25 can be excluded because these variables remained unchanged throughout the study. Our study was conducted in a well-controlled manner using a homogenous group of patients to minimize possible confounding factors.
Mechanisms
The mechanisms by which pravastatin affects proteinuria remain undefined. However, several factors, such as hemodynamics and lipid profiles, can be excluded. Regarding hemodynamics, systolic and diastolic blood pressures were similar in the two groups at baseline and during follow-up, providing evidence that pravastatin slows the decline in protein excretion through a renoprotective effect independent of changes in blood pressure. Glorioso et al26 showed hemodynamic improvement after 16 weeks of therapy with pravastatin in hypertensive patients, which was not consistent with our stable hemodynamics throughout the study in pravastatin-treated patients. The discrepancy could be due to differences in protocols, patient population, and periods of treatment. In fact, statins have been shown to decrease elevated but not normal blood pressure.27 Abnormalities of lipid metabolism are extremely common in a variety of renal diseases, which promote the acceleration of renal damage.28 Correction of hyperlipidemia may ameliorate renal injury. However, our results showed a poor correlation between the changes of lipid profiles and proteinuria, suggesting that other, nonlipid factors may be important in causing proteinuria regression. This result was compatible with previous findings of Park et al,29 who showed that statins improve renal function independent of cholesterol changes.
In this study, although pravastatin did not reduce plasma ET-1 levels, renal ET-1 production assessed by urinary ET-1 excretion significantly declined, implying the importance of the local renal ET-1 effects of pravastatin. Urinary ET-1 and plasma ET-1 are 2 distinct functional systems, each of which is regulated by its own control mechanisms. Because plasma ET-1 is produced by a great variety of normal cell types, including endothelial cells, neurons, the guts, and renal cells, plasma ET-1 levels may be affected by renal or nonrenal diseases. However, urinary ET-1 seems to be mainly derived from the amount of ET-1 locally produced in the kidney.30 Thus, although ET-1 is removed by the kidney, it has been suggested that urinary ET-1 reflects kidney renal synthesis rather than removal from the circulation. Pravastatin can be selectively taken up by the kidney.31 It is not surprising that renal ET-1 synthesis can be inhibited by pravastatin administration.
A substantial decrease in proteinuria as a result of inhibiting urinary ET-1 levels is suggested by the close correlation between ET-1 levels and proteinuria. ET-1 has been consistently implicated as playing a pivotal role in the pathogenesis of proteinuria, which in turn stimulates the tubular synthesis of ET-1. ET-1 stimulates both glomerular and interstitial fibroblast proliferation and has potent chemotactic effects on monocytes,32 which would further amplify inflammatory reaction. Transgenic mice overexpressing the ET-1 promoter have shown to develop renal glomerular and tubular fibrosis.33 Selective or nonselective blocking of ET receptors reduced proteinuria.9 The patients in the present study had glomerular and tubular dysfunction, as shown by a significantly higher excretion of IgG and retinol-binding protein compared with normal controls, implying that protein excretion in urine can result from a loss of the glomerular barrier function and a decrease of the tubular protein reabsorption. This is consistent with previous reports of impaired glomerular and tubular function in hypertensive patients with proteinuria.34 Glomerular function remained unchanged during treatment. A larger reduction in urinary retinol-binding protein excretion occurred after pravastatin administration, implying an improvement in the tubular properties. Thus, the pravastatin-induced reduction in proteinuria is mediated by improvement in tubular reabsorption rather than modifications in glomerular function.
Pravastatin further improved proteinuria in patients treated with AR blockers, suggesting that renal ET-1 may play a role in the pathogenesis of proteinuria in an angiotensin stimulation–independent manner. Previous studies have demonstrated anatomical distribution differences between ET-1 and angiotensin II type 1 receptors in the kidneys.35,36 Angiotensin II type 1 receptors are predominantly located in the glomeruli,35 in contrast to the distribution of ET-1, which is mainly located in tubular epithelial cells and, to a lesser extent, in glomeruli.36 It is not surprising that losartan has been shown to reduce proteinuria by improvement of glomerular basement membrane characteristics.37 Pravastatin, by attenuating ET-1, regressed proteinuria by improving tubular function in this study. However, whether anatomical distribution explains the synergistic effect of the combination of losartan with pravastatin on proteinuria remains unclear. In any case, these findings suggest that the underlying renoprotective mechanisms between AR blockers and pravastatin may be different. We think that our proposed mechanisms are reasonable and consistent with the notion that simultaneous blockade of AR and ET-1 activity was more renoprotective than either drug alone.38
In the placebo groups, we found no effect of losartan on proteinuria. This is not in line with previous findings5 showing that AR blockers slow the progression rate of renal disease in patients with proteinuria. Differences in study design and patient selection may explain the discrepancy.
Perspectives
The present study demonstrates that pravastatin administration was associated with further reduction of urinary protein excretion mediated by reducing renal ET-1 production and improving tubular function in patients with well-controlled hypertension, independent of blood pressure and lipid changes. Hypertensive patients with persistent proteinuria under intensive antihypertensive therapy have shown a significantly higher prevalence of end-organ damage.3 In view of the correlation between proteinuria progression and subsequent renal impairment, it is not unrealistic to anticipate a beneficial effect on clinical events in our population. This finding may provide a new strategy to treat persistent proteinuria in patients with well-controlled hypertension and may be of particular importance for the future design of combination drugs.
Pravastatin was, in part, a generous gift from the Sankyo Company, Tokyo, Japan. A special acknowledgment is made to the participants for their cooperation and commitment to the trial.