

 2021-09-02
2021-09-02
INTRODUCTION
Common warts are treated by different physical modalities like electrocautery, cryosurgery and different lasers with variable results, and recurrences are very common. In the past, intralesional bleomycin was found to be very effective in treating warts particularly in periungual and palmo-plantar areas.[1–4] Bleomycin has an antitumor, antibacterial and antiviral activity which may be related to its ability to bind with deoxyribonucleic acid (DNA), causing bleomycin strand scission and elimination of pyrimidine and purine bases.[5] The bleomycin hydrolase enzyme which is known to inactivate bleomycin is normally found in all the body tissues but it is present in very small amounts in skin. Thus, after injecting it intralesionally, a significant amount of the active drug is available for the action at the site, and so even a small amount is enough for treatment of warts.[5] We conducted a placebo-controlled trial of the intralesional injection of bleomycin in Indian patients to know its efficacy in palmo-plantar and periungual warts which are usually difficult to treat with other modalities.
MATERIALS AND METHODS
The present clinical trial was conducted between June 2009 and March 2010 in the Department of Dermatology. A written informed consent was taken from all the patients and ethical clearance was obtained from appropriate authorities of the college. Fifty patients with multiple palmo-plantar and periungual warts of more than 3-month duration, without any history of previous treatment, whose age ranged from 15 to 40 years were included in the present study. This age group was selected because multiple warts are common in this age group and these patients can be easily be counselled for follow-up. Pregnant women and patients with a history of Raynaud's disease or any other systemic illness were excluded from the study. Diagnosis was made on the basis of history and typical clinical features. Patients were categorized into two groups (groups A and B) of 25 each. Alternate patients were included in groups A and B which were treated with intralesional bleomycin and normal saline (placebo), respectively.
Bleomycin for injection is available in vials containing 15 mg powder. It was diluted first with 5 ml distilled water to prepare the stock solution which can be stored for 60 days at 4–8°C. Two parts of 2% lignocaine and one part of the bleomycin stock solution was taken in a tuberculin syringe, so that the final concentration became 1 mg/mL. Each wart and the adjacent skin was cleansed with isopropyl alcohol. Superficial paring was done to remove the callus surrounding the wart; bleeding points were not reached and the fresh solution was injected strictly intralesionally till blanching of the lesion occurred [Figure 1]. The amount of the injection given depends on the size of warts: warts up to 5 mm, 10 mm and more than 10 mm received 0.2 mL, 0.5 mL and 1.0 mL, respectively. The total volume injected at one treatment sitting was limited to 2 mL, and the injection into a single wart was limited to 1 mL. After 2 weeks of bleomycin injection, a black, ecchymosed eschar developed which was pared, and residual warts if present were injected a second time. In the controlled group, normal saline was injected in a similar manner as the bleomycin solution. The patients were followed up weekly for first month, fortnightly up to 3 months and then quarterly up to 1 year. A chi-square test was applied for statistical analysis using M-stat software. Routine haemogram, liver function tests, renal function tests and X-ray of the chest were done before and after 3 months of treatment.
![]()
Blanching of the wart after intralesional bleomycin injection
RESULTS
One hundred and fifty-seven warts (157) were present in 50 patients which were included in the present study. There were 27 males and 23 females whose mean age was 26.1 years and 29.2 years, respectively. Group A and B patients were having 85 and 72 warts, respectively [Table 1]. The baseline parameters (age, sex, sub-distribution of warts) between the two groups were statistically comparable, and no significant statistical difference was observed. A total of 82 (96.47%) of the 85 warts treated with intralesional bleomycin in a dosage of 1 mg/mL in group A showed complete resolution after one or two injections within 12 weeks. The response in periungual warts was 100%, while the response in palmo-plantar warts was 96.10% [Table 2]. A total of 25 of the 85 warts in group A required a second injection. Nonresponders from the control group were treated later with intralesional bleomycin (1 mg/mL) injection. In all the patients, ecchymosis was taken as the primary outcome at the first visit. Warts regressed without any scarring but with a slight pigmentation that gradually faded during the follow-up of 1 year [Figure 2, Figures Figures3a3a–b]. None of the resolved wart recurred at the end of the 1-year follow-up. Of 72 warts treated with normal saline (group B), only 8 (11.11%) showed disappearance of the lesion within 3 months [Table 2], but on further follow-up 19 (26.38%) warts disappeared at the end of 1 year. The difference in the resolution rate at the end of 12 weeks was statistically highly significant (P = 0.001) between groups A and B. The ecchymosed eschar did not develop in warts treated with normal saline. There was no change in haematological or biochemical investigation after 3 months of treatment in both groups. Most patients in both groups experienced moderate pain during injection that was of short duration and similar in both groups. There was no effect on nail growth or nail dystrophy following intralesional injection around the nail. None of the patients experienced any systemic toxicity.
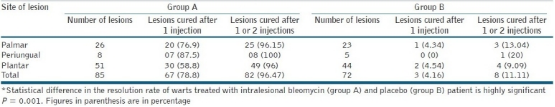
Table 1
Number of patients and lesions at different sites in each group
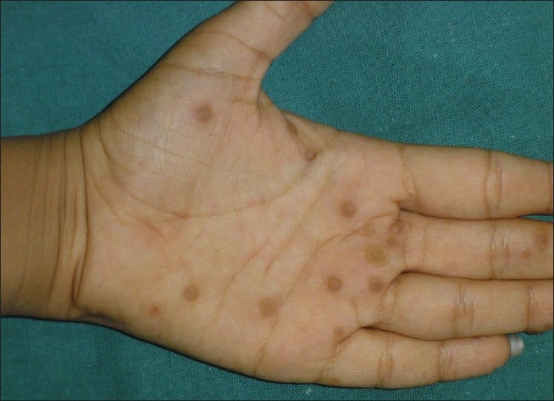
Table 2
Response of warts at different locations in Groups A and B after 12 weeks
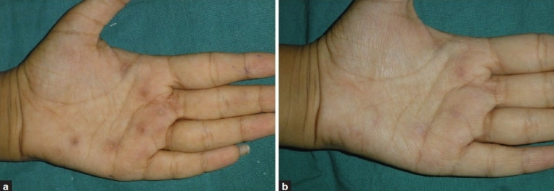
Multiple palmar warts before the initiation of treatment
(a) Partial improvement at the second week; (b) Complete resolution of warts without atrophy and hyperpigmentation after 6 weeks of intralesional bleomycin
DISCUSSION
Common warts have been a frustration for both patients and clinicians since early Greek and Roman times. They can greatly affect patient's quality of life by causing embarrassment, fear of negative appraisal by others and frustration caused by persistence and/or recurrence.[6] Warts may fail to clear even after repeated treatment with various physical modalities, and in the past, different studies have shown variable results with electrodissection,[7] cryosurgery[8] and different types of lasers.[9,10] Previous studies on the intralesional bleomycin use have shown excellent results particularly in plantar and periungual warts.[5,11,12] The use of intralesional bleomycin has not been approved by the US FDA. Bleomycin when used in high doses (>450 units) as in cancer chemotherapy can cause pulmonary fibrosis. For a very low dose (1 mg/mL), no systemic side effects have been observed.[13] We determined the efficacy of bleomycin in the treatment of warts at periungual and palmo-plantar sites which are difficult to treat with other modalities. Our results have shown a 96.10% cure rate in palmo-plantar warts which is higher than that (87%) in a similar study conducted by Salk and Douglas.[11] In 1983, Shumer and O’Keefe conducted a double-blind placebo-controlled study and treated 151 warts with intralesional bleomycin and 55 warts with normal saline as placebo. Their study showed a 60% cure rate for plantar warts and a 94% rate for periungual warts.[5] The present study has shown a 96.10% clearance rate of palmo-plantar warts with intralesional bleomycin which is much higher than that in the above-mentioned study. This may be due to the effect of paring which was done before injecting the second dose of intralesional bleomycin. The cure rate of periungual warts was almost similar in both the studies. None of the placebo-treated warts cleared in the study conducted by Shumer and O’Keefe while our study has shown an 11.11% cure rate at the end of 12 weeks. Olson reported the resolution of plantar warts in 18 of 25 (72%) patients with bleomycin and 5 of 21 (27%) patients treated similarly with placebo[14] while our study showed a cure rate of 96.47% and 11.11% for the warts treated with bleomycin and normal saline as placebo, respectively, in 12 weeks. Dermojet was used by Olson in his study, which can cause scattering of the solution and results in too small a dose being delivered within warts, which led to lower cure rates in the study.[14] Hayes and O’Keefe reported a cure rate of 78%, much lower than that in the present study; this may be due to the lower concentration of bleomycin used in their study.[15] Bremner treated 142 warts in 24 patients with intralesional bleomycin and reported a 63% cure rate which is lower than that in our study.[1] The multiple-puncture technique using a bifurcated vaccination needle to introduce bleomycin in warts used by Shelly and Shelly in a study also mentioned a success rate of 92%.[16] Potential side effects include scarring, change in pigmentation, nail damage and Raynaud's phenomenon were observed in few studies.[17,18] Concentrations of bleomycin, such as 0.15% and 0.05%, had also been used to treat warts.[19] Our results have shown bleomycin to be highly effective in the treatment of palmo-plantar and periungual warts. The stock solution prepared in our study was more concentrated than the previous studies, and hence it was diluted with 2% lignocaine which also worked as a local anaesthetic and reduced localized pain during and after injection.
CONCLUSION
Intralesional bleomycin injection was significantly safer and effective, with better patient acceptance in treating warts, particularly in palmo-plantar and periungual regions.