

 2021-08-19
2021-08-19
Case Report
Sevelamer Carbonate Crystal-Induced Colitis
![]()
![]()
1Community Memorial Health System, Graduate Medical Education, Ventura, CA, USA
2Community Memorial Health System, Graduate Medical Education, Department of Internal Medicine, Pacific Inpatient Physicians, Ventura, CA, USA
3Community Memorial Hospital, Department of Pathology, Ventura, CA, USA
4Community Memorial Hospital, Department of General Surgery, Ventura, CA, USA
Correspondence should be addressed to T. Lai; thaihnglai@gmail.comReceived19February2020;Accepted30March2020;Published24July2020 Academic Editor: Olga I.Giouleme
Copyright © 2020 T. Lai et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Hyperphosphatemia is a common and well-described complication of end-stage renal disease. Despite strict dietary constraints and compliance, phosphate binders such as calcium acetate and/or sevelamer carbonate are also needed to treat secondary hyperparathyroidism.Thiscasevignettedescribesanunderrecognizedadverseeffectofaphosphatebinder,sevelamercarbonate, inducing colitis in a 47-year-old male with insulin-dependent diabetes complicated by end-stage renal disease. He presented for recurrent abdominal pain with associated nausea and was found to have multiple circumferential lesions on computed to- mography including distal ascending, transverse, and proximal descending colon. Colonoscopy demonstrated nearly obstructing lesions worrisome for colonic ischemia or inflammatory bowel disease. Pathological review of histology demonstrated ragged colonicmucosawithulcerativedebrisandnonpolarizingcrystallinematerialatthesitesofulceration,morphologicallyconsistent with the phosphate binder, sevelamer carbonate. Sevelamer carbonate was discontinued, and the patient was transitioned to calcium carbonate with strict dietary restrictions. His symptoms improved with the cessation of sevelamer, and he was sub- sequently discharged home. He eventually underwent renal transplant without redevelopment of symptoms. Recognition of this underreported complication of sevelamer carbonate, phosphate binder, is of utmost importance in directing appropriate therapy with cessation of this medication in the setting of gastrointestinal complaints or more specifically enteritis and colitis. Clinicians providing care to end-stage renal patients taking either sevelamer and/or sodium polystyrene sulfonate should have increased awarenessofthepossiblegastrointestinalsideeffects.
End-stage renal disease (ESRD) is associated with multiple metabolic and electrolyte derangements. Hormonal and electrolyte imbalance of calcium, phosphorus, and PTH can result in short-term complications such as calciphylaxis and long-term sequelae such as renal osteodystrophy, increased cardiovascular events, and all-cause mortality [1–3]. As a result, many patients have strict dietary constraints, and despite compliance, phosphate binders such as calcium acetate and/or sevelamer carbonate are also needed to treat secondary hyperparathyroidism [1,2]. Of the two more commonly used, sevelamer carbonate is a calcium-free phosphate binder that is composed of a nonabsorbable resin
andhasbeenconsideredoneofthepreferredagentsbyNKF KDOQI guidelines [4,5]. It has many common gastroin- testinalsideeffectsincludingnausea,vomiting,constipation, and abdominal pain that limit patient compliance [2,6].These symptoms previously had no specific etiology, but recently, sevelamer carbonate is receiving recognition as a possiblecauseofmucosalinjury[6–8].Theexactmechanism of injury remains elusive, but in the last 5 years, Swanson et al. described that variable degrees of mucosal injury throughout the gastrointestinal tract from “gum to bum” are believed to be associated with sevelamer use. These include acute and chronic inflammation, ulceration, and features of ischemia with mucosal necrosis from samples obtained via endoscopy/colonoscopy[7].
2 Case Reports in GastrointestinalMedicine
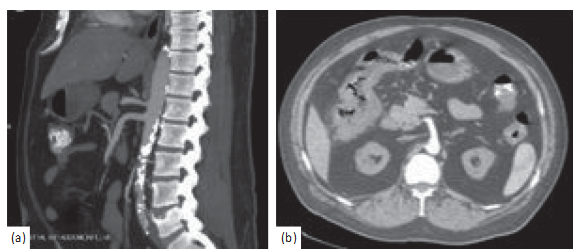
Figure1:Computedtomographyangiogramwithcontrastoftheabdomenandpelvisdemonstratingatheroscleroticdiseaseofthe abdominalaortawithoutinvolvementofmajorarterialsupplyoftheintestine(a)andpericolicfatstrandingwithwallthickeningoftheright hepaticflexure(b).

Figure2:Imagesfromcolonoscopydemonstratingmultiplelesionsincludingobstructingcircumferentiallesionintheascendingcolon(a,
b) and circumferential but nonobstructing lesion in the transverse colon (c).
A 47-year-old male with hypertension and insulin-depen- dent diabetes complicated by end-stage renal disease pre- sentedwitha2-dayhistoryofcrampyabdominalpain.Itwas associated with nausea and anorexia but without additional gastrointestinal symptoms such as diarrhea or hema- tochezia. He had a similar episode 1 month prior with workup including computed tomography imaging followed by colonoscopy, notable for numerous circumferential lesions.
Biopsy histology was suspicious for colonic ischemia, and there were no histologic features of inflammatorybowel disease. It was suspected this constellation of symptoms could have developed as sequelae to hypotension during hemodialysis while on antihypertensive medications. His antihypertensivemedicationswerediscontinued,andhewas provided supportive care with nonoperative management. He had resolution of his pain and was able to tolerate diet and was discharged home. He returned one month later for recurrence of abdominal pain and nausea. He was empiri- cally started on antimicrobials and underwent repeat im- agingwiththecomputedtomographymesentericangiogram that demonstrated only moderate features of atherosclerotic diseasewithpatentceliac,superiormesentericartery(SMA), and inferior mesenteric artery (IMA) (Figure 1(a)).Addi- tional findings included features of colitis with pericolicfat
stranding in the distal ascending, transverse, and proximal descending colon (Figure 1(b)). He underwent repeat co- lonoscopy showing similar findings (3 regions of circum- ferential ulceration in the ascending/hepatic flexure, splenic flexure, and descending colon) (Figures 2(a)–2(c)). Biopsy histology demonstrated ragged colonic mucosa with ul- cerative debris and nonpolarizing crystalline material at the sites of ulceration (Figures 3–5). Clinical workup for ad- ditionalcausesincludingcardioemboliceventsandvasculitis was completed and found to be nondiagnostic. Histology from the current and prior colon biopsy specimens dem- onstrated crystalline material morphologically consistent with the phosphate binder sevelamer carbonate. Sevelamer carbonate was discontinued, and the patient was transi- tioned to calcium carbonate with strict dietary restrictions. His symptoms improved with the cessation of sevelamer, and he was subsequently discharged home. He eventually underwent renal transplant without redevelopment of symptoms.
This vignette illustrates a rare and likely underrecognized adverse effect of therapy with sevelamer carbonate in the treatment of hyperphosphatemia in patients with end-stage renal disease. Review of the literature shows a limited number of reported cases (estimate of about 50 cases) and
Case Reports inGastrointestinalMedicine 3
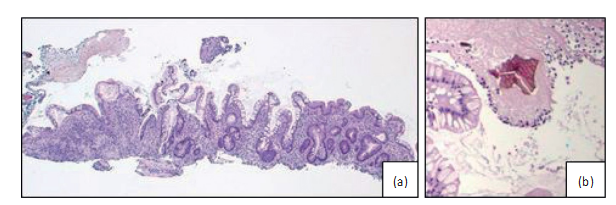
Figure3:(a)Raggedcolonicmucosafromthecircumferentiallesionsbiopsiedduringcolonoscopy.(b)Ahighpowerviewofasevelamer crystalonhematoxylinandeosin-stained(H&E)slidewithcharacteristic“fishscale”morphologyandyellow/goldcolor.Bothsevelamer (Figure3(b))andsodiumpolystyrenesulfonatehavesimilar“fishscale”morphologyandarenonpolarizablebypolarizedlightmicroscopy. TheprimarymorphologicdifferencebetweenthesetwocrystaltypescanbeseenonstandardH&E-stainedslides.Sevelamercrystalscanbe distinguishedbyyellow/goldcolorwhencomparedwithsodiumpolystyrenesulfonatecrystals,whichshowdarkvioletstaining.
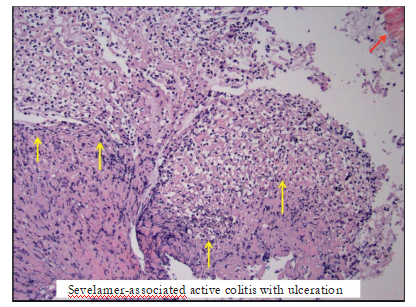
Figure4:Imagefromhistologydemonstratingulceratedcolonicmucosawithadherentfibrinousexudate(yellowarrows)inthepresenceof sevelamercrystals(redarrow)seeninthetoprightcorner.

Figure5:Imagefromhistologydemonstratingactivecolitis(yellowarrows)againinthepresenceofsevelamercrystals(redarrow)as previouslyseen,topleftcorner.
illustrates the immense range of presenting symptoms and possible pathology identified. This condition is likely more common, but underrecognized as there are many reasonsfor
patientswithend-stagerenaldiseasetohavegastrointestinal complaints.Additionally,therelativerarityofcomplications with severity to prompt further evaluation is complicatedby
4 Case Reports in GastrointestinalMedicine
nonspecific colonoscopic findings and potentially subtle histopathologic features, which may not include crystals dependent on biopsy sampling. It is also possible that the pathologyismissedasaresultofmisidentificationorfailure to identify crystals from resin-based medications like sev- elamer and sodium polystyrene sulfonate[9–13].
Although not previously described, there are clinical circumstanceswhenpatientswithESRDmaychronicallybe taking more than one medication associated with mucosal injury. This creates a diagnostic dilemma, in which the diagnosis may solely rely on the histopathologic review of tissue and identification by crystal morphology. As previ- ously discussed by Gonzales et al. in their review and comparison of classic features of medication resins, seve- lamer carbonate morphology was described as flaky (com- monly described as “fish scales”), rectangular in shape,with hues of pink (central) with peripheral transition to yellow/ orange pigment by H&E staining[9].
Recognition of this underreported complication of sevelamer carbonate, phosphate binder, is of utmost im- portance in directing appropriate therapy with cessation of this medication in the setting of gastrointestinal complaints ormorespecificallyenteritisandcolitis.Cliniciansproviding care to end-stage renal patients taking either sevelamer and/ or sodium polystyrene sulfonate should have increased awareness of the possible gastrointestinalside effects.
Patient consent was obtained prior to submission.
The authors declare no conflicts of interest.