

 2021-08-19
2021-08-19
Efficacy and Tolerability of Sevelamer Carbonate in Hyperphosphatemic Patients Who Have Chronic Kidney Disease and Are Not on Dialysis
Markus Ketteler,* Marianne Rix,† Stanley Fan,‡ Nicholas Pritchard,§ Ove Oestergaard,ǁ Scott Chasan-Taber,¶ Jeremy Heaton,¶ Ajay Duggal,¶ and Philip A. Kalra**
Design, setting, participants, & measurements: This was an open-label, dosage-titration study. Patients with serum phos- phorus >5.5 mg/dl were enrolled (n = 46). Sevelamer carbonate was administered for 8 wk. Patients were supplemented with native vitamin D (400 IU). The primary efficacy parameter was the change from baseline in serum phosphorous. Secondary measures included the percentage of serum phosphorus responders; changes in serum lipids, calcium-phosphorus product, and bicarbonate; and safety and tolerability.
Results: Sevelamer carbonate treatment resulted in a statistically significant decrease in mean serum phosphorous levels from baseline to end of treatment. A total of 75% of patients with stage 4 and 70% of patients with stage 5 chronic kidney disease achieved the target serum phosphorous at the end of treatment. There were statistically significant decreases in serum calcium-phosphorus product and total and low-density lipoprotein cholesterol at the end of treatment and a statistically significant increase in mean serum bicarbonate levels (from 16.6 to 18.2 mEq/L). Sevelamer carbonate was well tolerated.
Conclusions: Sevelamer carbonate is an effective and well-tolerated therapy for the control of phosphorous levels in hyperphosphatemic patients who have chronic kidney disease and are not on dialysis.
Clin J Am Soc Nephrol 3: 1125–1130, 2008. doi: 10.2215/CJN.05161107
![]() n early to moderately advanced chronic kidney disease (CKD), serum phosphorus levels are maintained at near- normal levels by compensatory enhanced phosphorus ex- cretion (fibroblast growth factor-23, parathyroid hormone [PTH]) by the residual nephrons, resulting in preservation of net phosphorus excretion. As renal failure progresses, the GFR decreases, resulting in the loss of preservation or balance of net phosphorus excretion and the subsequent development of hy- perphosphatemia. There is considerable experimental and clin- ical evidence indicating that hyperphosphatemia is associated with a number of deleterious consequences, such as secondary hyperparathyroidism, arterial calcification, and renal osteodys-
n early to moderately advanced chronic kidney disease (CKD), serum phosphorus levels are maintained at near- normal levels by compensatory enhanced phosphorus ex- cretion (fibroblast growth factor-23, parathyroid hormone [PTH]) by the residual nephrons, resulting in preservation of net phosphorus excretion. As renal failure progresses, the GFR decreases, resulting in the loss of preservation or balance of net phosphorus excretion and the subsequent development of hy- perphosphatemia. There is considerable experimental and clin- ical evidence indicating that hyperphosphatemia is associated with a number of deleterious consequences, such as secondary hyperparathyroidism, arterial calcification, and renal osteodys-
Received November 20, 2007. Accepted February 19, 2008.
Published online ahead of print. Publication date available at www.cjasn.org.
Correspondence: Prof. Markus Ketteler, Nephrologische Klinik, Klinikum Coburg gGmbH, Ketschendorfer Strasse 33, 96450 Coburg, Germany. Phone:
+49-9561-249611; Fax: +49-9561-249612; E-mail: markus.ketteler@klinikum- coburg.de
trophy (1–3). Hyperphosphatemia has also emerged as one of the more important risk factors for mortality in dialysis pa- tients, and this association reproducibly seems to show concen- tration-dependent characteristics in epidemiologic studies (4). Recent studies demonstrated that incident dialysis patients already carry a significant cardiovascular disease burden and show a high prevalence of vascular calcifications (5). Moreover, patients in advanced stages of CKD have higher risk for death than of reaching the dialysis stage (6). It is unclear whether dialysis modalities in themselves change the pathophysiologic consequences of hyperphosphatemia, but it is certainly more likely that the adverse effects of hyperphosphatemia are inde- pendent of the dialysis status of a patient with CKD. The data showing the clinical consequences of hyperphosphatemia in patients who have CKD and are not on dialysis are less abun- dant because this population has not been closely studied, but both Kestenbaum et al. (7) and Voormolen et al. (8) found an association between serum phosphate level and mortality in patients who had CKD and were not on dialysis. This suggests
that it is correct to consider that these patients are subject to the harmful effects of hyperphosphatemia similar to those seen in patients who are on dialysis.
As in patients who are on dialysis, the management of hy- perphosphatemia is based initially on limiting phosphate in- take through appropriate dietary measures. Failure to control serum phosphorus adequately by these means should be fol- lowed by pharmacologic intervention. Phosphate binder ther- apy is advocated for the treatment of hyperphosphatemia in patients who have CKD and are not yet on dialysis by a number of guidelines, including the widely accepted National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) guidelines on bone and mineral metabolism (9). The currently available phosphate binders are sevelamer hydrochloride (Renagel; Genzyme Corporation, Cambridge, MA), lanthanum (Fosrenol; Shire US Inc., Wayne, PA), calcium acetate (PhosLo; Fresenius Medical Care North America, Waltham, MA), calcium carbonate, and aluminum hydroxide. There are no recently published studies of phosphate binders in patients who had CKD and were not on dialysis, and none of the phosphate binders has an indication specifically for the treatment of hyperphosphatemia in patients who have CKD and are not on dialysis.
Sevelamer carbonate (Renvela; Genzyme Corporation, Cam- bridge, MA) has been developed as an improved, buffered form of sevelamer hydrochloride (Renagel). Sevelamer carbonate is an anion exchange resin with the same active moiety as sevel- amer hydrochloride in which carbonate replaces chloride as the anion. The replacement of the chloride with carbonate provides bicarbonate ions that may be a benefit to patients who have CKD and are not receiving dialysis, who are prone to acidosis and do not receive the benefits of renal replacement therapy. Sevelamer carbonate has been found to have the same safety and efficacy profile as sevelamer hydrochloride in hemodialy- sis patients (10). This study was designed to investigate the effects of sevelamer carbonate on the control of serum phos- phorus levels, calcium-phosphorus product, lipids, and bicar- bonate in hyperphosphatemic patients who had CKD and were not on dialysis.
Patients at 19 nephrology centers in Northern Europe and Australia who were aged Š18 yr, had a serum phosphorus level Š5.5 mg/dl either at screening (for patients who were not on a phosphate binder) or after washout (for patients who were taking a phosphate binder), and had 25-hydroxy vitamin D measurement of Š10 ng/ml and an intact PTH (iPTH) measurement of Š800 pg/ml were eligible for the study. Patients were excluded from the study when they had a severe gastrointestinal motility disorder, poorly controlled diabetes, hyperten- sion, or any other clinically significant unstable medical condition.
All patients signed written, informed consent before the initiation of any study-related activities. The protocol was reviewed and approved by an institutional review board. This research was carried out in accordance with Good Clinical Practice guidelines, applicable regula- tions, and the ethical principles that have their origin in the Declaration of Helsinki.
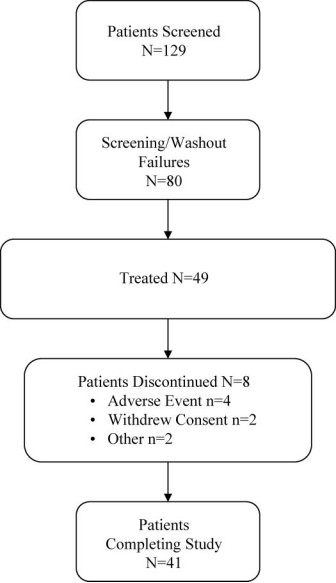
Figure 1. Patient disposition.
This was a multicenter, open-label, single-arm, dosage-titration study of hyperphosphatemic patients who had CKD and were not on dialysis. The study consisted of four periods: A 2-wk screening period, a 2-wk washout period for patients who had previously been on a phosphate binder, an 8-wk treatment period, and a second 2-wk washout period. Patients who had a serum phosphorus level Š5.5 mg/dl either at screening (patients not on phosphate binders) or after washout (pa- tients on previous phosphate binders) and met all other eligibility criteria entered the 8-wk treatment period. The starting dosage of sevelamer carbonate was 4.8 g daily (2 × 800-mg tablets three times daily). The sevelamer carbonate dosage was titrated at biweekly inter- vals to a maximum dosage of 12 g/d (15 × 800-mg tablets) during the treatment period in increments of 2.4 g/d (1 × 800-mg tablet three times daily) to attain a target serum phosphorus level (Š2.7 and Š4.6 mg/dl for patients with stage 4 CKD and Š5.5 mg/dl for patients with stage 5 CKD). Patients were supplemented with a daily dose of 400 IU of the native form of vitamin D at bedtime during the treatment period.
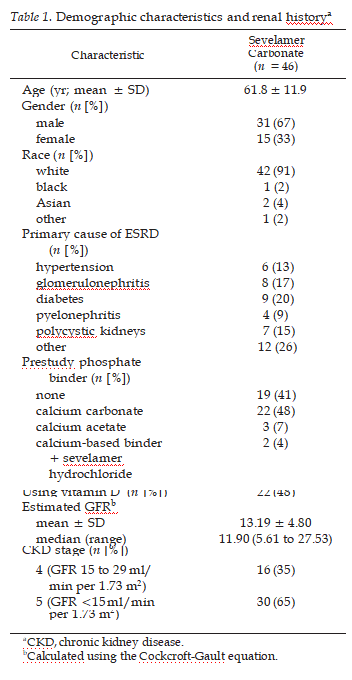
A second phosphate binder washout phase followed the active treat- ment period to establish the degree of efficacy, after which patients were returned to their previous therapy. Throughout the study, pa- tients were not to make any intentional changes in their diet and were not to be started on treatment with 1,25 dihydroxyvitamin D and/or cinacalcet or lipid-lowering medications. If prescribed these medica- tions before study initiation, then the dosage was to be maintained.