

 2021-08-18
2021-08-18
Sevelamer hydrochloride is a cationic, polymeric ion-exchange resin derived from poly(allylamine hydrochloride) crosslinked with epichlorohydrin. Although not absorbed in the gut, sevelamer may swell to 6–8 times its molecular weight when exposed to the fluids of the gastrointestinal tract.
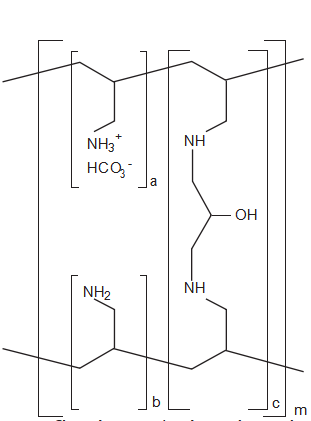
Figure 2. Chemical structure of sevelamer carbonate. a, b = number of primary amine groups, where a + b = 9; c = number of crosslinking groups, where c = 1; m = large number to indicate an extended polymer network. (From reference 19.)
In this state, each gram of drug can bind approximately 2.5–2.7 mmol of phosphate, with maximal binding occurring between pH values of 6–8.
Sevelamer is excreted from the body in the feces.2 It binds phosphate anions through ion exchange and hydrogen bonding. It is a nonabsorbable, noncalcium and nonaluminum binder that releases one hydrochloric acid molecule for every phosphate-bound molecule.17 This binding differs from that of calcium-, aluminum-, or lanthanum-based products, all of which form generally irreversible bonds.20
Diffusion controls ion exchange on the sevelamer resin; therefore, other anions in the gastrointestinal tract could displace phosphate anions, releasing them into the gut for absorption. Bile acids, for example, form a group of anions to which sevelamer can bind. However, because sevelamer possesses a finite number of anion-binding sites, bile acids may be bound at the expense of phosphorus, a situation that consequently diminishes the agent’s phosphate- binding potency.13 Several studies have found that sevelamer hydrochloride also decreased low- density lipoprotein cholesterol levels.21, 22 This positive effect on the lipid profile occurs by binding these bile acids similar to cholestyramine, but this effect may come at the expense of its phosphate-binding capacity.
Sevelamer hydrochloride contains 17% hydrochloric acid by weight, which is exchanged for phosphate and other anions in the intestinal tract. This nonselective anion-binding mechanism contributes to the major drawback of its formu- lation. Sevelamer hydrochloride disassociates in the acidic environment of the stomach and gastrointestinal tract, exchanging the chloride ions attached to its polymer backbone for phosphate ions (Figure 3).20 The resulting absorption of chloride ions has been associated with a concomitant decrease in serum bicarbonate concentration.20 Although the clinical significance of this decreased concen- tration has not been reported, the KDOQI clinical practice guidelines recommend that serum bicarbonate concentration be maintained above 22 mEq/L and that treatment should begin once the concentration falls below this level.8 However, of interest, most patients from the studies we reviewed had mean baseline levels
below 22 mEq/L, which were lowered even further when they were treated with sevelamer hydrochloride.
Several small studies of patients undergoing hemodialysis revealed a decrease in their serum bicarbonate concentrations and the development of metabolic acidosis with the use of sevelamer hydrochloride. For instance, in a retrospective study of 24 patients receiving hemodialysis, sevelamer hydrochloride significantly decreased serum bicarbonate concentrations from a mean ± SD of 20.02 ± 1.43 mEq/L before treatment to
17.89 ± 2.30 mEq/L (p=0.02) after treatment.15 The concentration differences in the control group receiving calcium- and aluminum- containing binders were not significant. Of interest, the study demonstrated no correlation between serum bicarbonate concentrations and doses of sevelamer hydrochloride.
By contrast, a retrospective study conducted in Japan revealed a statistically significant negative correlation between serum bicarbonate concentration and both the daily dose and the cumulative dose of sevelamer hydrochloride.18 Seventy-two patients receiving hemodialysis were started on doses of 750–1500 mg/day, which were gradually increased to 1500–6000 mg/day. Serum bicarbonate concentrations fell from a mean ± SD of 19.05 ± 1.69 mEq/L to 17.61 ± 1.59 mEq/L
(p<0.001). This finding suggests that the serum bicarbonate level reductions among patients taking sevelamer hydrochloride may be a dose- dependent effect.
Two other studies, one from Greece16 and the other from Italy,17 demonstrated statistically significant decreases in serum bicarbonate concentrations among hemodialysis-dependent patients taking sevelamer hydrochloride. Both were prospective comparisons of sevelamer hydrochloride versus a control. For 2 years, the Greek investigators tracked 14 patients who were stabilized with hemodialysis and either sevelamer hydrochloride or a calcium-based regimen.16 The authors reported that serum bicarbonate concentrations in the sevelamer group were significantly lower early and remained lower than those observed in the calcium-based group
throughout the study (p<0.01). Results were a bit difficult to interpret, as the researchers never reported the mean serum bicarbonate concen- trations for either group. Data were instead presented graphically and did appear to show significantly lower bicarbonate concentrations with sevelamer hydrochloride compared with the calcium-based regimen, especially at 3 and 12 months. At the end of the study, differences between the groups no longer appeared to be statistically significant, although the reason for the change in bicarbonate concentration was not discussed. Bicarbonate concentrations in the dialysate, however, were maintained throughout the study. Therefore, in the group receiving sevelamer hydrochloride, serum bicarbonate concentrations clearly declined during the study period.
The Italian investigators enrolled a total of 16 patients in two groups.17 Each group began therapy with either sevelamer hydrochloride or calcium carbonate; after 24 weeks, they switched to the other agent. The serum bicarbonate concentration was a mean ± SD of 21.1 ± 0.7 mEq/L in patients taking calcium carbonate compared with 17.3 ± 1.1 mEq/L in patients taking sevelamer hydrochloride (p<0.01).
In the United States, the Calcium Acetate Renagel Evaluation (CARE) study of 100 patients receiving hemodialysis likewise showed that patients receiving sevelamer hydrochloride had lower serum bicarbonate concentrations than those receiving calcium acetate.10 During 8 weeks of treatment, mean concentrations were 20.4–21.9 mEq/L with calcium acetate and 19.2–20.2 mEq/L with sevelamer (p<0.0001). During weeks 2–8, the percentage of patients with serum bicarbonate concentrations below 22 mEq/L ranged from 45.7–65.2% in the calcium acetate group and 72.3–86.7% in the sevelamer hydrochloride group (p<0.0001). Of note, the effect on serum bicarbonate level was not the main outcome measure of the study, which was primarily designed to measure the efficacy of calcium acetate and sevelamer hydrochloride in controlling and maintaining target phosphorus levels and the calcium phosphorus product.
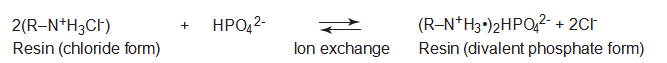
Figure 3. Chemical binding of resins to phosphorus through the ion-exchange process. Weakly basic resins are most effective when the pH is below 9. R = polymer matrix. (From reference 20.)
However, these findings concerning serum bicarbonate concentrations agreed with those of the European studies and further implicated sevelamer hydrochloride as the culprit.
A few studies have examined metabolic acidosis due to sevelamer hydrochloride in patients requiring peritoneal dialysis. In one multicenter trial of 228 patients, serum bicarbonate concentrations below 22 mEq/L were observed more frequently in patients receiving sevelamer hydrochloride compared with other agents, and these concentrations were generally lower with sevelamer hydrochloride than with other drugs (mean ± SD 24.0 ± 3.2 vs 26.7 ± 3.2 mEq/L,
p<0.01).23 The frequency of metabolic acidosis was 22% of 128 sevelamer-treated patients versus 5% of 100 patients receiving other agents.
Investigators who retrospectively evaluated 163 patients undergoing peritoneal dialysis observed metabolic acidosis (serum bicarbonate concentration < 24 mEq/L) in 13.5%.24 Serum bicarbonate concentrations were lower in patients receiving sevelamer than in those receiving calcium carbonate (mean ± SD 24.5 ± 2.7 vs 26.2 ± 2.3 mEq/L, p<0.01).
Other small investigations of sevelamer hydrochloride in patients requiring peritoneal dialysis yielded conflicting results. One study of 35 patients, 18 of whom were taking sevelamer hydrochloride, found serum bicarbonate concentrations below 22 mEq/L in only three.25 Although the sample size was small, the authors concluded that sevelamer hydrochloride produced no detectable effect on serum bicarbonate concen-trations. Of note, patients were receiving automated peritoneal dialysis, lactate-buffered continuous ambulatory peritoneal dialysis, or bicarbonate and lactate–buffered continuous ambulatory peritoneal dialysis, which could have affected their serum bicarbonate concentrations.
Another study of 89 patients receiving peri- toneal dialysis, 12 of whom were taking sevelamer hydrochloride, uncovered no metabolic acidosis; however, serum bicarbonate concentrations were significantly lower among the patients taking sevelamer (mean 26.33 vs 27.97 mEq/L, p=0.05).26 The authors suggested that peritoneal dialysis provides continuous buffering against major fluctuations in serum bicarbonate concen- trations because of the high lactate or bicar- bonate levels in peritoneal dialysis solutions.
Evaluated together, the results of all these studies seem to suggest that sevelamer hydro- chloride has the same potential to produce metabolic acidosis in patients receiving either
peritoneal dialysis or hemodialysis. However, buffered dialysate solutions are continually exchanged on a more regular basis with peri- toneal dialysis than with conventional hemo- dialysis, which may mitigate the risk for devel- oping metabolic acidosis from sevelamer hydrochloride.