

 2021-08-19
2021-08-19
The effects of treatment on serum phosphorus measurements, total cholesterol, LDL cholesterol, HDL cholesterol, and serum calcium (al- bumin adjusted)-phosphorus product were analyzed using the change from baseline to the end of the treatment period for the intention-to- treat population. A Wilcoxon signed rank test was used to assess these changes. The proportion of patients who attained serum phosphorus
response (serum phosphorus Š2.7 and Š4.6 mg/dl for patients with stage 4 CKD and Š5.5 mg/dl for patients with stage 5 CKD) was also tabulated.Safetywasevaluatedonthebasisofthefrequencyofadverse
events and changes in laboratory parameters. The study was designed to have 80% power to detect a 1 mg/dl mean change from baseline in
serum phosphorus assuming an SD of 1.4 mg/dl and a type I error rate of 0.05. All statistical analyses were performed in SAS 8.2 (SAS Insti- tute, Inc., Cary, NC) in a validated environment.
Atotalof129individualpatientswerescreenedforthis study (Figure 1). Of these, 80 (62%) were not eligible for the study, mostly because of phosphorus levels <5.5 mg/dl. Forty-nine patients were treated, and the 46 patients who had a serum phosphorus measurement after the initiation of study medica- tion were included in the analysis. Baseline demographic char- acteristics and renal history are presented in Table 1. Fifty-nine percent of patients were on a phosphate binder and required a washout before initiating sevelamer carbonate treatment. Con- comitant medications are reflective of those commonly used by patients who have CKD and are not on dialysis. The most common classes of medication prescribed as concomitant ther- apy were vitamin D and analogues (78%), sulfonamides plain (67%), non-iron bivalent anti-anemic oral preparations (61%) and hepatic hydroxymethyl glutaryl–CoA reductase inhibitors (57%). The patients included in this study had a wide range ofGFR (calculated using the Cockcroft-Gault equation)values,andinmanypatients,therenalclearanceoverlappedwiththe GFR level seen in dialysis patients (Figure 2).
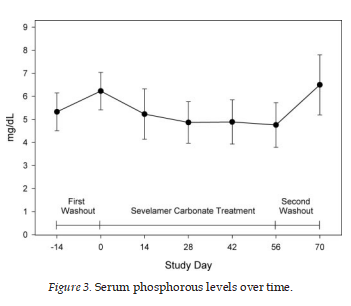
The mean serum phosphorus was 5.3 ± 0.8 mg/dl at screen- ing for patients who had previously been on a phosphate binder. At baseline, after the 2-wk phosphate binder washout for patients who had previously been on a phosphate binder or at screening for patients who had not previously been on a phosphate binder, the mean serum phosphorus was 6.2 ± 0.8mg/dl. After 8 wk of treatment, the mean serum phosphorus had decreased to 4.8 ± 1.0 mg/dl (Figure 3). Treatment with sevelamer carbonate resulted in a statistically significant mean decrease of 1.4 ± 1.0 mg/dl in mean serum phosphorous levels from baseline to end of treatment (P < 0.001). The meanserum phosphorus increased to 6.5 ± 1.3 mg/dl after the post-treat- ment washout period. By the end of the 8 wk sevelamer car- bonate treatment period, 75% of patients with stage 4 CKD had reached the titration target level of a serum phosphorus Š2.7 and Š4.6 mg/dl and 70% of patients with stage 5 CKD had achieved a serum phosphorus Š5.5 mg/dl.

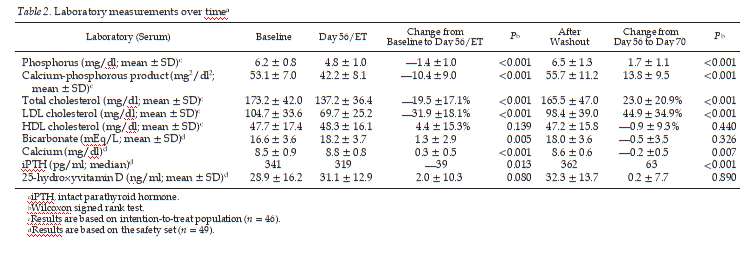
Serum lipids, calcium-phosphorus product, calcium, bicar- bonate, iPTH, and 25-hydroxyvitamin D levels over time are presented in Table 2. There were statistically significant de- creases in levels of serum calcium-phosphorus product, total cholesterol, and LDL cholesterol levels and an increase in se- rum calcium from baseline to the end of treatment (all P < 0.001). There was no clinically meaningful change in serum HDL cholesterol. There was a statistically significant increase in mean serum bicarbonate levels (1.3 mEq/L; range —4 to 8 mEq/L; P = 0.005). A total of 28 (61%) patients experienced an increase in bicarbonate levels. There was also a statistically significant reduction in iPTH from baseline to day 56/early termination (—39 pg/ml; P = 0.013) and an increase from
28.9 ± 16.2 ng/ml at baseline to 31.1 ± 12.9 ng/ml at day 56/early termination (P = 0.080) in mean serum levels of 25- hydroxyvitamin D.
The mean starting prescribed dosage was 4.8 g/d, as speci- fied by the protocol. The mean ending prescribed dosage after the dosage titrations during the study was 7.8 g/d. The mean actual daily dosage of sevelamer carbonate used in this study was 5.5 g (six to seven sevelamer carbonate 800-mg tablets per day) with a mean compliance of 89%.
Overall, sevelamer carbonate was well tolerated during the study.The highest frequency of adverse events was in minor to moderate gastrointestinal disorders. This is consistent with the known tolerability profile of sevelamer in dialysis patients, in whom adverse events involving gastrointestinal system are the most commonly observed. No serious adverse events or deaths that occurred during the study were considered to be related to treatment. Two patients withdrew from the study to begin dialysis treatment.
Discussion
In this study, the objective was to evaluate phosphorus re- duction in a population of patients who had CKD and were not
on dialysis and had serum phosphorus >5.5 mg/dl, a level widely considered to be a clinically meaningful level of hyper- phosphatemia (4). As in hemodialysis patients with serum phosphorus levels Š5.5 mg/dl, it is reasonable to assume that patients with earlier stage CKD will also be at risk from the harmful effects of this level of hyperphosphatemia. K/DOQI guidelines for patients with early-stage CKD recommend a phosphorus level to be in a lower range (2.7 to 4.6 mg/dl) than in patients with stage 5 CKD (<5.5 mg/dl), and there is mount- ing evidence that patients could benefit from reaching this lower level (7,8).
As expected, on the basis of previous studies of sevelamer in hemodialysis patients (11–15), sevelamer carbonate treatment re- sulted in statistically significant reductions in serum phosphorus. The mean serum phosphorus at the end of treatment was 4.8 mg/dl, a level well within the range of 3.5 to 5.5 mg/dl recom- mended for patients with stage 5 CKD (65% of the study popu- lation). Moreover, at least 70% of patients were able to achieve a serum phosphorous within the ranges recommended for CKD patients (Š2.7 and Š4.6 mg/dl for patients with stage 4 CKD; Š5.5 mg/dl for patients with stage 5 CKD). This response rate is slightly higher than the 50% response rate that would be expected on the basis of similar studies in the hemodialysis population and from large scale cross-sectional studies (16).
Because of the anticipated difficulty in recruiting hyperphos- phatemic patients who have CKD and are not on dialysis and have serum phosphorus levels Š5.5 mg/dl, a single-arm design whereby patients in this trial acted as their own controls, using pre- and post-treatment washout periods, was used. The in- crease in the serum phosphorus levels that were seen during the post-treatment washout period verified that the reductions that were seen during treatment were due to drug effects and not random chance.
Unlike other phosphate binders, sevelamer also binds bile acids. As shown in previous studies in hemodialysis patients (11–15), significant reductions in total and LDL cholesterol (—20 and —32%, for total and LDL cholesterol, respectively) were also found in this study. These reductions occur regard- less of the concomitant use of other lipid-control measures. Because K/DOQI guidelines (which are aligned with National Cholesterol Education Program guidelines) place patients with CKD in the highest cardiovascular risk category and recom- mend LDL <70 mg/dl (17), these reductions in LDL cholesterol may possibly be of additional benefit to patients who have CKD and are not on dialysis.
Acidosis has been associated with adverse effects on bone metabolism (18) and increased malnutrition and inflammation
(19) in HD patients. Oral bicarbonate supplementation was found to result in fewer hospital admissions and fewer days hospitalized in peritoneal dialysis patients (20). Acidosis is commonly seen in patients with CKD, and these effects may be of more concern in the predialysis patients, for whom the dialysis modality itself is not being used to correct these abnor- malities. The increase in serum bicarbonate seen with sevel- amer carbonate therapy may therefore confer an increased ben- efit over treatment that is acid-base neutral or potentially acidifying.
Previous studies (21,22) suggested that sevelamer treatment may be associated with reductions in 25-hydroxyvitamin D as a result of absorption of the vitamin from dietary sources. To ensure standardization of vitamin D status, patients in this study were supplemented with 400 IU of native vitamin D. Possibly as a result of this supplementation with vitamin D, the 25-hydroxyvitamin D level increased during sevelamer treat- ment in this study, and small increases were also seen in serum calcium. A small decrease in iPTH from 341 to 319 pg/ml (P < 0.013) was also noted. This is consistent with the effect of the small rises in serum calcium and increase in the levels of vitamin D concentrations that occurred during the study. Sig- nificant reduction of iPTH would be expected to reduce phos- phaturia and increase serum phosphorus. Thus, the reduction of serum phosphorus concentrations seen in this study is all the more significant and impressive.
Overall, sevelamer carbonate was well tolerated. The safety profile of sevelamer carbonate in this study is similar to the known safety profile of sevelamer hydrochloride in hemodial- ysis patients. The adverse events seen were consistent with previous findings and the underlying characteristics of the patients with CKD who made up this population.
Conclusions
Results of this study demonstrated that sevelamer carbonate was both efficacious in controlling serum phosphorus and well- tolerated in patients who had CKD and were not on dialysis. Statistically significant reductions in levels of calcium-phos- phorous product, total cholesterol, and LDL cholesterol were also observed, whereas serum calcium levels remained in the normal range. The increase in serum bicarbonate with sevel- amer carbonate treatment may be helpful in ameliorating the acidosis that is typically seen in patients who have advanced CKD and are not receiving dialysis.
Acknowledgments
This study was presented as an abstract at the annual meeting of the American Society of Nephrology; San Francisco, CA; October 31– November 5, 2007.
Disclosures
None.