

 2021-08-18
2021-08-18
Comparison of Sevelamer Hydrochloride and Sevelamer Carbonate: Risk of Metabolic Acidosis and Clinical Implications
Ashwini B. Pai, Pharm.D., and Brian M. Shepler, Pharm.D.
Hyperphosphatemia is highly prevalent in patients with chronic kidney disease (CKD), particularly in those with advanced or end-stage renal disease. Sevelamer hydrochloride is an ion-exchange resin that reduces serum phosphorus concentrations. The agent also produces favorable lipid profile effects and does not cause hypercalcemia. However, reported drawbacks of this agent are metabolic acidosis, high pill burden, and a relatively low affinity and selectivity for phosphate anions. Sevelamer carbonate is a new buffered formulation that does not increase the risk of metabolic acidosis. To determine the roles of these two agents in the treatment of hyperphos- phatemia in patients with CKD, we performed a MEDLINE search (June 1995–June 2008) focusing on the mechanism of action of resin binding with phosphate and the development of metabolic acidosis. We also reviewed studies that evaluated the effects of sevelamer hydrochloride or sevelamer carbonate on serum bicarbonate concentrations. Several studies in patients with CKD and hyperphosphatemia who received hemodialysis or peritoneal dialysis found decreases in serum bicarbonate concentrations with the use of sevelamer hydrochloride, whereas sevelamer carbonate did not have this negative effect on bicarbonate concentrations. Both drugs appear to be equivalent in their abilities to lower serum phosphorus concentrations. However, as sevelamer carbonate does not decrease serum bicarbonate levels, it may be more appropriate for patients at risk for metabolic acidosis who require phosphate binders that do not contain calcium or aluminum.
Key Words: sevelamer hydrochloride, sevelamer carbonate, metabolic
acidosis, chronic kidney disease, hyperphosphatemia.
OUTLINE
Mechanism of Action and Pharmacology of Nonabsorbable Exchange Resins
Risk of Metabolic Acidosis with Sevelamer Hydrochloride
Studies of Sevelamer Carbonate Implications for the Clinician Conclusion
From the Department of Pharmacy Practice, School of Pharmacy and Pharmaceutical Sciences, Purdue University, West Lafayette, Indiana (both authors).
Address reprint requests to Brian M. Shepler, Pharm.D., Department of Pharmacy Practice, School of Pharmacy and Pharmaceutical Sciences, Purdue University, 575 Stadium Mall Drive, West Lafayette, IN 47907-2091.
Hyperphosphatemia is highly prevalent in patients with chronic kidney disease (CKD)— particularly in those with advanced or end-stage renal disease—and it has emerged as one of the most important risk factors for mortality among patients receiving dialysis.1 In early-to-moderate CKD, compensatory mechanisms maintain serum phosphorus concentrations at near-normal levels. As renal failure progresses, decreasing glomerular filtration rates impair the excretion of phosphorus. About 600–700 mg of phosphorus must be excreted every 24 hours to maintain phosphorus homeostasis.2
As the glomerular filtration rate decreases to below 20 ml/minute, it becomes difficult for the
urinary excretion of phosphorus to match dietary intake. High serum concentrations of phosphorus promote its precipitation with serum calcium to form calcium phosphate calcifications. These precipitates are transported in the blood and are deposited in tissues throughout the body, a process called soft tissue calcification. Calcifi- cation of heart muscle can increase rigidity of arterial walls, leading to arteriosclerosis and premature heart disease, whereas calcium- phosphate deposits in the cardiac valves can contribute to conduction abnormalities. Vascular and cardiac calcifications are serious compli- cations resulting from untreated hyperphos- phatemia and can increase a patient’s cardio- vascular risk.3–7
Clinicians routinely measure serum calcium and phosphorus concentrations in patients with CKD to monitor for hyperphosphatemia. In addition, such measurements can be used to calculate another monitoring parameter: the calcium-phosphorus product. This parameter is used to predict an individual patient’s risk for developing soft tissue calcification, with a value above 55 mg2/dl2 suggesting increased risk.8
Hyperphosphatemia is also one of the mechanisms that promote release of parathyroid hormone from the parathyroid gland. This situation leads to secondary hyperparathyroidism in many patients with CKD. In most cases, serum phosphorus concentrations should be normalized to below 4.6 mg/dl so that drug therapy to reduce parathyroid hormone secretion can be maximally effective.8
In most cases, dietary restrictions alone are insufficient to restore phosphorus homeostasis. Intermittent (thrice-weekly) hemodialysis corrects high serum phosphorus concentrations only partially and not to the extent necessary to reach current treatment goals in the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI).8 At present, primary treatment of hyperphosphatemia in patients with CKD is reduction of phosphorus concentrations by using dietary restrictions and phosphate-binding agents.
Currently available pharmacotherapeutic agents for hyperphosphatemia include calcium- and aluminum-containing phosphate binders, lanthanum carbonate, and ion-exchange resins. Calcium-containing phosphate binders— primarily calcium carbonate and calcium acetate—are the treatments most widely used because of their efficacy and low cost. In a study comparing a calcium-free phosphate binder with
calcium carbonate, hypercalcemia was associated only with calcium carbonate,9 although a similar study using calcium acetate did not reproduce this finding.10
Aluminum-containing phosphate binders used in patients with impaired renal function are associated with a heightened risk of toxicity, resulting in diarrhea, osteomalacia, and neurologic and hematologic complications.2, 11
Lanthanum carbonate has shown promising results with its ion-exchange mechanism and ability to bind high levels of phosphate. It may also increase adherence by decreasing pill burden and appears to retain its efficacy across a broad range of pH values.12, 13
Sevelamer hydrochloride, a nonabsorbable hydrogel, is an ion-exchange resin that reduces serum phosphorus concentrations (Figure 1).14 The agent also produces favorable lipid profile effects and does not cause hypercalcemia. However, reported drawbacks of this agent are metabolic acidosis, high pill burden, and a relatively low affinity and selectivity for phosphate.11, 15–18 Sevelamer carbonate (Figure 2) is a new buffered formulation that does not increase the risk of metabolic acidosis.19 As such,
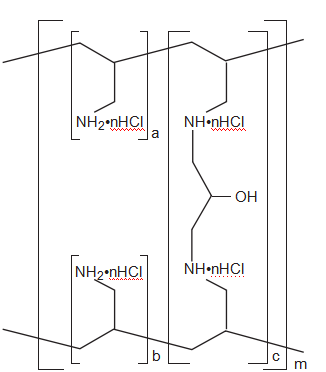
Figure 1. Chemical structure of sevelamer hydrochloride. a, b = number of primary amine groups, where a + b = 9; c = number of crosslinking groups, where c = 1; n = fraction of protonated amines, where n = 0.4; m = large number to indicate an extended polymer network. (From reference 14.)
it may be a better treatment alternative than sevelamer hydrochloride for patients who require sevelamer therapy.
To determine the roles of sevelamer hydro- chloride and sevelamer carbonate in the treatment of hyperphosphatemia in patients with CKD, we performed a MEDLINE search (June 1995–June 2008) focusing on the mechanism of action of resin binding with phosphate and the development of metabolic acidosis. Search terms were sevelamer hydrochloride, sevelamer carbonate, and metabolic acidosis. We also reviewed studies that evaluated the effects of sevelamer hydrochloride or sevelamer carbonate on serum bicarbonate concentrations.