

 2021-08-18
2021-08-18
Calcium Polycarbophil
Calcium polycarbophil (Figure 1) is a white to creamy white, very fine powder with a slight
Table 2. Comparative “Water-holding” Capacity of Hydrophilic Substances5
Agent | Synthetic Intestinal Juice | Synthetic Gastric Juice |
Agar-agar | 14 | 11 |
Carob gum | 22 | 14 |
Psyllium | 30 | 28 |
Methylcellulose | 36 | 13 |
Polycarbophil3 | 120 | 12 |
aThe calcium salt of polycarbophil comprises approxi mately 20 percent calcium; hence, the "water-holding" capacity is reduced to about 100 (70-105) ml per gram.6 The United States Pharmacopeia7 requires that 1.0 g of polycarbophil absorb not less than 62 g of sodium bicar bonate solution.
ester-like odor. Like its parent compound, it is insoluble in water, dilute acids, and dilute alka lis.4 The material possesses exceptionally high water-binding capacity,5 7 is not absorbed,15 does not interfere with the activity of digestive enzymes16 or intestinal absorption,15 possesses satisfactory stability,4 is physiologically inert, and does not cause gastrointestinal irritation.6-17 Tagged resin appears unaltered physically or chemically in the stool of rats.15 Calcium polycar- bophil possesses little chelating ability and thus does not remove essential metabolites, vitamins or minerals, even when fed in effective doses for long periods of time.2 5 It has negligible ion-ex change capacity for sodium, being on the order of 1-3%.6 Because of its superior hydrophilic capacity and inert physical and physiological properties, calcium polycarbophil should be use ful for the symptomatic management of both con stipation and diarrhea.5 As the water-retaining capacity of calcium polycarbophil is consider ably greater than that of methylcellulose or psyl lium mucilloid, it may prove superior to these agents in modifying fecal consistency.5
Bulk agents that form colloidal suspensions in dilute acids, or show only a modest differential of hydrophilia in dilute acids and alkalis, have proven unsatisfactory for use in pediatric pa tients, which have the greatest frequency of diar rheal problems. Polycarbophil suspensions are not palatable; calcium polycarbophil, in contra distinction, forms a palatable aqueous suspen sion, which possesses virtually no hydrophilic ac tivity. After oral administration, the calcium is replaced by hydrogen ions from gastric hydro chloric acid, and the polycarbophil acid may
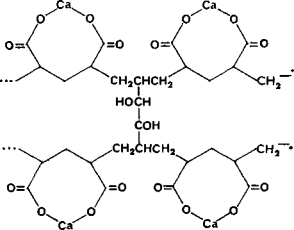
Figure 1. Structural formula of calcium polycarbophil.
then exert its maximal hydrophilic action on reaching the alkaline medium of the small intes tine and colon.14 Presumably, individuals with achlorhydria might find the product consider ably less effective.
Calcium Load
When calcium polycarbophil is administered as a laxative or antidiarrheal agent, the exchange of calcium for hydrogen ions permits ionized cal cium to become available for absorption. An in crease in plasma Ca++ may be causally related to increased gastrin release from depots (G-cells) in the pyloric antrum, resulting in increased gas tric hydrochloric acid secretion. After an oral dose of calcium polycarbophil of 1.5 g to chil dren or 5.0 g to adults, 300 to 1,000 mg of cal cium will become available for absorption. Theo retically, this could be harmful, particularly if the calcium polycarbophil were administered for a long length of time.
The usual daily intake of calcium from food sources in children and adults is 3 to 20 mg/kg/ day. Daily calcium requirements are about 10 mg/kg/day, but good health can be maintained within a wide range of daily calcium intake be cause of adaptive mechanisms.18
Absorption of calcium ion is homeostatically controlled at the gut level by a complex process under the influence of hormones, vitamins, and other factors.19-21 The intestinal regulation of cal cium absorption is one mechanism by which high dietary loads of calcium do not cause eleva tion of calcium plasma levels above the normal range; the amount absorbed is tn a range that can be excreted in the urine without precipita tion. The ability of bone to take up rapidly plasma calcium also assists in maintaining the normal level of plasma calcium.18 An intake of less than one-fifth the daily requirement may be associated with pathological states, but intake of excess calcium ion has not been associated with systemic or local derangements tn otherwise healthy subjects.18-22 Major medical and pediatric references do not describe health hazards associ ated with excess calcium ion intake; ingestion of large quantities of a calcium salt is unlikely per se to cause hypercalcemia except in patients with hypothyroidism.23 Limitation of calcium in take is recommended in a few disease states such as idiopathic hypercalciuria and milk-alkali syndrome, but even in these conditions, restric tions, while usually imposed, may prove to be impractical because of the universal availability of calcium.24
Relative Equivalence of Polycarbophil and Calcium Polycarbophil
The chemical equivalence of polycarbophil and calcium polycarbophil is readily determined
because of the stoichiometric amount of calcium found in calcium polycarbophil. Considered on an anhydrous basis, calcium constitutes 20% of calcium polycarbophil. Thus, 1 g of calcium poly carbophil contains 0.2 g of calcium and 0.8 g of polycarbophil; or conversely, 1 g of polycarbo phil is equivalent to 1.25 g of anhydrous calcium polycarbophil.
While the stoichiometric relationship is clear from the chemical equivalence viewpoint, the physical parameter of hydrophilia may not show a parallel relationship between polycarbophil and calcium polycarbophil. The hydrophilic prop erties of calcium polycarbophil are regularly monitored during preparation of the pharmaceu tical product to ensure batch-to-batch unifor mity. The ability to absorb water is dependent upon pH, the length of the polymer chain, and the degree of cross-polymerization; in the in vitro test, the degree of water absorption is also de pendent on the particle size of the material.
Owing to the greater resiliency or elasticity of polycarbophil compared with calcium polycarbo phil, the latter can be milled to finer particles. Because of this, the in vitro test7 probably does not reflect the in vivo situation. In the latter cir cumstance, the particles are discretely dispersed throughout the gastrointestinal tract, whereas in the in vitro assessment the particles are packed in a container and the "measured'' amount of water absorbed consists of that truly absorbed by the resin plus that amount of water entrapped in the interstices between particles. Since cal cium polycarbophil is milled to a finer particle size, the interstitial spaces are smaller, allowing for less contribution to the apparent hydrophilic ability of the material. The water absorption test is primarily to ascertain that a proper degree of cross-linking has occurred in the polymerization reaction.
In the Final Order for Antacid and Antiflatulent Products Generally Recognized as Safe and Ef fective and Not Misbranded,25 the following is stated:
"Calcium-containing active ingredients: Cal cium as carbonate or phosphate; maximum daily dosage limit 160 mEq calcium (e.g., 8 grams cal cium carbonate)."
One hundred sixty milliequivalents of calcium is equal to 3.2 grams of calcium, the maximum daily dosage limit. If the recommended dosage schedule for calcium polycarbophil is followed, the amount of calcium in the recommended daily dose will be as given in Table 3. Thus, the addi tional amount of calcium in the recommended dose of calcium polycarbophil, 1.44 g, is less than half the maximum 3.2 g permitted in the O-T-C monograph for antacids and has a satis factory margin of safety even at the maximum recommended dosage level.
No warning for calcium at any specified dos age level, as has been stated for sodium, potas sium and magnesium, was recommended by the O-T-C antacid panel or the O-T-C laxative and antidiarrheal panel; the O-T-C vitamin-mineral- hematinic panel has permitted the following daily allowances and permissible ranges for die tary supplements with respect to calcium: infants —0.6 g; children under4 — 0.8 g (0.125 to 1.2 g); adults — 1.0 g (0.125 to 1.5 g).
Thus, the amount of calcium released from cal cium polycarbophil taken at the recommended dosage level should not be considered a poten tial hazard.4