

 2021-04-08
2021-04-08
Introduction
Cardiovascular disease is now recognized as one of the leading causes of morbidity and mortality not only in adults but also in children with chronic kidney disease (CKD). Indeed, several studies have shown a high prevalence of cardiovascular risk factors, such as vascular calcifications and left-ventricular hypertrophy across the spectrum of CKD in children. Although this process is multifactorial, the use of calcium-based phosphate binders has been associated with the development of vascular calcifications in adult and pediatric patients receiving dialysis. Sevelamer hydrochloride (HCl), on the other hand, has been shown to be a safe and effective phosphate binder, providing the same control of skeletal lesions associated with secondary hyperparathyroidism as calcium-based binder therapy. Furthermore, sevelamer HCl has been associated with decreased progression of vascular calcifications in adult patients undergoing hemodialysis
However, the use of sevelamer HCl has been associated with a dose-dependent metabolic acidosis in both pediatric and adult dialysis patients. This side effect is concerning in children, as chronic metabolic acidosis is considered a potential risk factor for poor growth. Sevelamer carbonate is a similar anion exchange resin in which chloride is replaced by carbonate. It has been shown to be equally effective in lowering phosphorus, with a reduced incidence of metabolic acidosis in adult patients on dialysis. Therefore, this study was designed to assess the effects of sevelamer carbonate on acid-base status in pediatric dialysis patients.
Material and methods
The patient population was comprised of children receiving University of California-Los Angeles (UCLA) Medical Center. This study was approved by the UCLA Institutional Review Board, and all patients/parents gave informed consent to participate. Patients were enrolled in a prospective 3-month trial with the following inclusion criteria: a minimum of 3 months on automated peritoneal dialysis or hemodialysis, sevelamer HCl as primary phosphate binder, a serum bicarbonate <20 mmol/L, or the need for sodium bicarbonate supplementation. Upon entry into the study, sevelamer HCl was replaced by an identical dose of sevelamer carbonate, and the following biochemical variables were determined every 4 weeks: phosphorus, calcium, intact parathyroid hormone (iPTH), complete blood cell count, serum glucose, sodium, potassium, chloride, bicarbonate, magnesium, creatinine, urea, albumin, and alkaline phosphatase. For patients on sodium bicarbonate supplementation, blood pressure (BP) and interdialytic weight gain (IDWG) were recorded before and every month after starting sevelamer carbonate. Results are reported as systolic and diastolic BP indexes (average BP divided by the 95th percentile for age, sex, and height). Data is presented as medians with a 25−75 percentile range. Comparisons between groups were made using nonparametric tests (KruskalWallis test and Dunn’s posttest for comparisons of more than two groups, and Wilcoxon signed rank test for two-group comparisons). Ap value of <0.05 was considered significant. outpatient peritoneal or hemodialysis at the Ronald Reagan.
Results
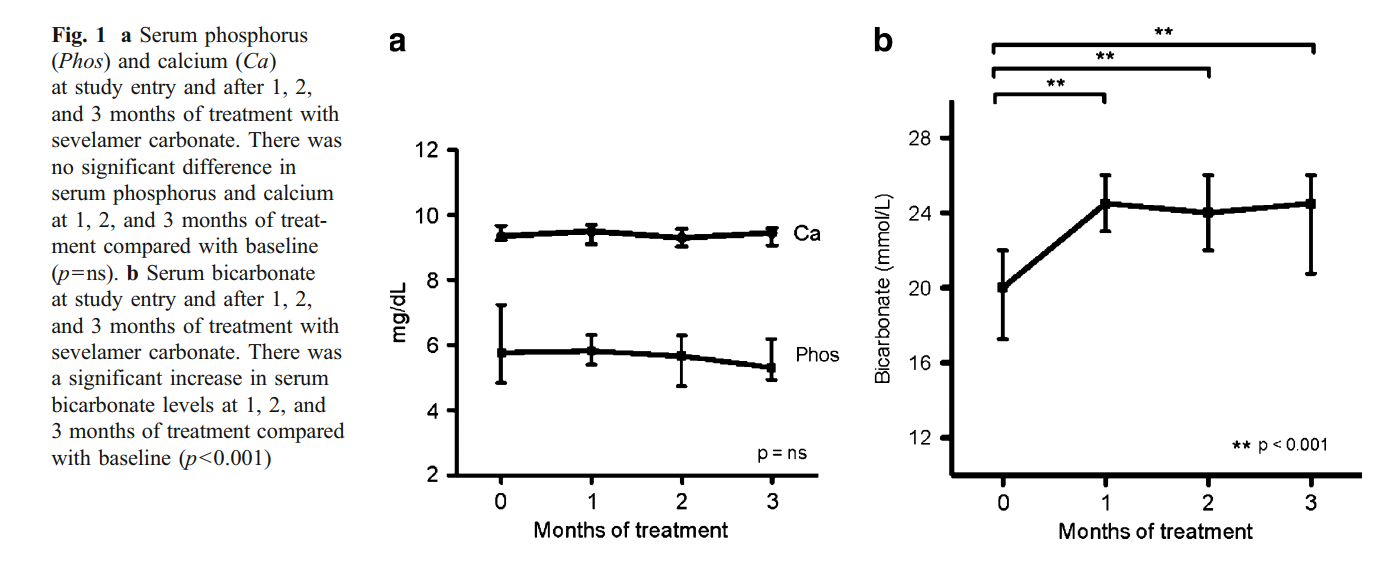
A total of 24 patients aged 16±3 years were enrolled in the study. Mean duration of dialysis therapy for the ten patients receiving hemodialysis and 14 receiving peritoneal dialysis was 9±13 months. At study entry, the dose of sevelamer HCl was 11.9±3.8 g/day. Six peritoneal dialysis and four hemodialysis patients were receiving sodium bicarbonate supplementation (average of 2.3±1 g/day). Twelve patients were treated with active vitamin D sterol therapy. Baseline serum calcium and phosphorus levels were 9.3 (9.2–9.7) mg/dl and 5.7 (4.8–7.2) mg/dl, respectively, and remained stable during the follow-up period (Fig. 1a). Intact PTH was 330 (136– 668) pg/ml at the beginning of the study and 510 (258–1051) pg/ml after 3 months (p=0.076). Initial serum bicarbonate levels were 20.0 (17.2–22.0) mmol/L (20.5 mmol/L in peritoneal dialysis vs. 20 mmol/L in hemodialysis patients; p=ns). Levels increased to 24.5 (20.75–26) mmol/L at the end of the study (p<0.001) (25 mmol/L in peritoneal dialysis and 23 mmol/L in hemodialysis patients; p=ns) (Fig. 1b)
All sodium bicarbonate supplementation was discontinued by 2 months in the ten patients previously on alkali therapy. Among these ten patients, BP and antihypertensive therapy remained unchanged. Systolic and diastolic BP indexes at the beginning of the study were 0.93 (0.85–1) and 0.83 (0.78– 0.99), respectively. BP indexes remained stable throughout the study and were 0.87 (0.81–1.01) and 0.8 (0.65–0.94), respectively, after 3 months (p=ns). BP indexes were not different between peritoneal dialysis and hemodialysis patients. In addition, there was no difference in mean IDWG at the beginning of the study and after 3 months [1.1 (0.2–1.2) kg and 1.1 (0.8–1.4) kg, respectively; p=ns].
Discussion
The results of this study demonstrate that sevelamer carbonate is an effective phosphate binder in pediatric dialysis patients. Serum calcium and phosphorus remained unchanged after the switch from sevelamer HCl to sevelamer carbonate. The median serum iPTH increased after the switch, but this trend did not reach statistical significance. In addition, the change to sevelamer carbonate normalized serum bicarbonate levels, eliminating the need for alkali therapy previously required with sevelamer HCl.
Previous studies in both pediatric and adult patients have demonstrated that metabolic acidosis is a relatively common adverse side effect of sevelamer HCl. In particular, Pieper et al., in a crossover study of 18 pediatric CKD patients (17 dialysis dependent) showed that sevelamer HCl was as effective as calcium acetate in lowering phosphorus levels but was associated with a higher rate of metabolic acidosis (34.4% vs. 3.3%). Sevelamer carbonate, on the other hand, in a study of 79 adult hemodialysis patients, was equivalent to sevelamer HCl in controlling phosphorus and increased bicarbonate levels by 1.3±4.1 mEq/L over 2 months. Similar findings were described in adult patients with CKD not yet on dialysis.
The increase and normalization of serum bicarbonate levels after the switch to sevelamer carbonate is of particular importance in children with CKD in whom growth retardation remains a significant problem. It is well recognized that children with chronic metabolic acidosis and normal renal function have poor growth, and in vitro studies have shown that metabolic acidosis is associated with a decrease in bone mineralization. Thus, current Kidney Disease Outcomes Quality Initiative (K/DOQI) nutritional guidelines for children recommend maintaining a serum bicarbonate level >22 mmol/L. Furthermore, in adult dialysis patients, metabolic acidosis has been associated with an increased risk of death and hospitalizations
The improvement in acid-base status had the additional advantage of allowing the discontinuation of all sodium bicarbonate supplementation. This reduction in salt intake, on average 2.3 g/day, may have a beneficial effect in a population already fluid sensitive and prone to cardiovascular disease. In our study, there was no change in the IDWG, BP medications, or BP during the follow-up period. This study may have been underpowered to detect such differences, and larger trials are needed. However, discontinuation of sodium bicarbonate supplementation led to a decrease in the number of pills per day, which may have a positive impact in a population where noncompliance is an ongoing issue.
In conclusion, although sevelamer HCl and sevelamer carbonate are both effective non-calcium-based phosphate binders in pediatric dialysis patients, sevelamer carbonate has the additional advantage of maintaining serum bicarbonate within the normal range. The preferential use of sevelamer carbonate in pediatric CKD patients, including patients not yet on dialysis, could have a positive impact on growth, along with decreasing salt intake and the burden of medications.