

 2021-09-02
2021-09-02
Introduction
Infections caused by Gram-positive pathogens remain common, and resistance to traditional, established antibiotics is increasingly recognized. Daptomycin, a cyclic lipopeptide parenteral antibiotic, exhibits rapid concentration-dependent bactericidal activity against Gram-positive pathogens such as methicillin-resistant Staphylococcus aureus (MRSA).1 The in vitro bactericidal activity of daptomycin showed that the minimum inhibitory concentrations (MICs) at which 90% of clinically relevant Gram-positive bacteria tested were inhibited were ≤1 µg/mL, except for enterococci, for which the MICs were 2–4 µg/mL.2 Daptomycin has a distinct mechanism of action involving the calcium-dependent insertion of the compound into the bacterial cytoplasmic membrane.3,4 The interactions with the Gram-positive bacterial surface lead to rapid disruption of the membrane without penetrating into the cytoplasm or causing lysis.5 Daptomycin also inhibits the synthesis of protein, DNA, RNA, and lipoteichoic acid, and is effective at all growth phases including the stationary phase. This property may be particularly useful in the treatment of indolent and deep-seated infections, such as endocarditis and osteomyelitis, where bacteria may exist within biofilm.5
Daptomycin is approved for the treatment of complicated skin and soft tissue infections (cSSTIs) (4 mg/kg/day), right-sided infective endocarditis (RIE) caused by S. aureus, and bacteremia associated with cSSTIs or RIE (6 mg/kg/day).6 Daptomycin is not indicated for the treatment of pneumonia.7
Published data on pharmacokinetics, clinical safety and efficacy/effectiveness (including high dose and combination therapy), utility in outpatient parenteral antimicrobial therapy (OPAT), and drug resistance of daptomycin are summarized.
Pharmacokinetics of daptomycin
Daptomycin’s pharmacokinetics has been examined in healthy subjects and in patients in both single- and multiple-dose studies. Pharmacokinetics is generally linear and time independent at doses up to at least 12 mg/kg/day administered for 14 days (Figure 1).8 Following repeated once-daily doses, steady-state concentrations are achieved by the third dose. The serum half-life of the drug is 8–9 hours.9 Daptomycin is renally excreted, and its systemic clearance in healthy adults is 0.011 L/kg/h.10 Dosing must be adjusted for adult subjects who have impaired creatinine clearance.11,12 In patients with chronic kidney disease stage 4 (creatinine clearance <30 mL/min) and in subjects undergoing hemodialysis or peritoneal dialysis, a dosage adjustment of daptomycin is recommended.6,9 Daptomycin has a very small volume of distribution of ∼0.1 L/kg. It is distributed primarily to extracellular fluid, does not cross cell membranes, and is ∼92% reversibly bound to serum proteins.9,13
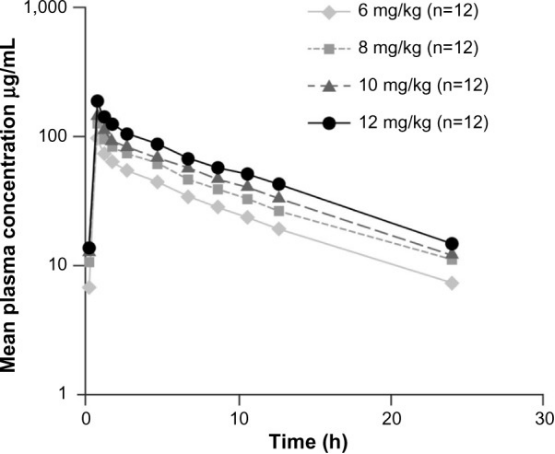
Plasma daptomycin concentration–time curves at steady state (day 4).
Notes: Antimicrob Agents Chemother, 2006;50(10):3245–3249. DOI: 10.1128/AAC.00247-06,8 reproduced with permission from American Society for Microbiology.
Pharmacokinetic studies of daptomycin have been reported in pediatric patients suggesting that higher doses of daptomycin may be required due to increased plasma clearance and hence lower area under the plasma concentration–time curve as compared with adults.14 In a recently completed study (NCT00711802) of daptomycin in patients aged 1–17 years with cSSTIs, age-adjusted daptomycin doses were administered once daily to achieve exposures demonstrated to be successful in adult studies of cSSTIs: 12–17 years, 5 mg/kg/day; 7–11 years, 7 mg/kg/day; 2–6 years, 9 mg/kg/day; and 1 to <2 years, 10 mg/kg/day.15 As described in the 2011 Infectious Diseases Society of America (IDSA) treatment guidelines, high-dose (6–10 mg/kg/day) daptomycin may be used in pediatric patients as an alternative drug for the management of MRSA bacteremia, infective endocarditis, acute hematogenous osteomyelitis, and septic arthritis.16
As shown in Table 1, the pharmacokinetics of daptomycin has been assessed in specific populations, such as critically ill patients and patients with morbid obesity, moderate hepatic failure and renal disease.11,17–21
Table 1
Pharmacokinetics of daptomycin in special populations
Authors | Patients | Daptomycin (dose, treatment duration) | Findings |
Dvorchik11 | N=19 with moderate hepatic failure; aged ≥18 years | 6 mg/kg once | No statistically significant differences in Cmax or AUC were found as compared to healthy volunteers. Adjustment in daptomycin dose or dose regimen was not required |
Dvorchik and Damphousse18 | N=25 patients with morbid obesity; aged ≥18 years | 4 mg/kg once | Cmax and AUC increased by 25% and 35%, respectively. There was no effect on half-life and no change in fraction of daptomycin excreted unchanged in the urine |
Di Paolo et al17 | N=58 with severe Gram-positive infections; aged ≥18 years | 4–12 mg/kg/day, 18 days | Daptomycin plasma concentrations lower than that described for healthy volunteers |
Corti et al19 | N=9 with continuous renal replacement therapy; aged ≥18 years | 6 mg/kg/day, 5 days | PK of daptomycin similar to healthy volunteers |
Khadzhynov et al20 | N=8 with continuous renal replacement therapy; aged ≥18 years | 8 mg/kg loading dose followed by 4 mg/kg every 48 hours* | PK of daptomycin similar to healthy volunteers |
Kullar et al21 | N=160 with renal impairment (23.8% on hemodialysis); aged ≥18 years | 5.8–7.8 mg/kg/day, 13.5 days | Successful outcome observed in 128 (80%) patients at the end of daptomycin therapy. Daptomycin was considered as a safe and effective therapeutic option in patients with renal insufficiency |
Note:
*Treatment duration is missing.
Abbreviations: AUC, area under plasma concentration–time curve; Cmax, maximum plasma concentration; PK, pharmacokinetics.
Safety studies of daptomycin
Daptomycin has generally been shown to have a good safety profile in several randomized clinical trials (Table 2).22–28 In two randomized, controlled, Phase III clinical trials in 1,092 patients with cSSTIs, daptomycin (4 mg/kg/day) therapy was compared with conventional antibiotics such as penicillinase-resistant penicillins.28 Daptomycin was well tolerated. The discontinuation rates were low and similar to standard therapy (2.8% in both groups). The most commonly reported adverse events (AEs) were constipation, nausea, and headache. In an open-label, randomized trial (246 patients) with daptomycin (6 mg/kg/day) use in bacteremia and endocarditis, similar overall incidence of AEs was observed in daptomycin and standard therapy groups.27 Clinically significant renal dysfunction was lower in patients who received daptomycin as compared to those who received standard therapy (11.0% and 26.3%, respectively, P=0.004).
Table 2
Characteristics of the main efficacy and safety studies for daptomycin
Authors | Patients | Daptomycin (dose, treatment duration) | Comparator (type, dose, treatment duration) | Efficacy results | Safety results |
Aikawa et al22 | N=101 patients with cSSTIs; aged ≥20 years | 4 mg/kg/day over 30 minutes, 7–14 days | Vancomycin 1 g over at least 60 minutes, twice daily, 7–14 days | Clinical success rate: 81.8% (95% CI: 69.1%, 90.9%) with daptomycin and 84.2% (95% CI: 60.4%, 96.6%) with vancomycin | AEs related to the study drug: 21.6% with daptomycin and 27.3% with vancomycin. Elevation of CPK >500 U/L found in one patient in each group. None of these CPK elevations led to study discontinuation or was serious |
Konychev et al23 | N=120 patients with cSSTIs; aged ≥65 years | 4 or 6 mg/kg/day over 30 minutes, 5–14 or 10–28 days with bacteremia | Semisynthetic penicillin 2 g every 6 hours or every 4 hours for patients with bacteremia; vancomycin 1 g every 12 hours, 5–14 or 10–28 days with bacteremia | Clinical success rate: 89.0% with daptomycin and 83.3% with pooled comparators; odds ratio 1.65 (95% CI: 0.49, 5.54) | Rates of AEs were similar between treatment groups: 62.5% with daptomycin and 65.0% with pooled comparators. AEs related to the study drug: 13.8% and 12.5%, respectively. Elevation of CPK was found in one patient in the daptomycin group and two patients in the pooled comparator group |
Quist et al24 | N=194 patients with cSSTIs; aged ≥18 years | 4 mg/kg/day, 4–10 days | Vancomycin 1 g twice daily; teicoplanin 400 mg once daily | Clinical success rate: 91.4% with daptomycin and 87.2% with the comparator | Similar incidences of AEs and serious AEs across treatment groups. Severe AEs: 7.2% with daptomycin and 16.3% with comparators. Discontinuation due to AEs or death: 3.1% and 9.8%, respectively. Elevation of CPK was found in one patient in the daptomycin group (no discontinuation) and in five patients in the pooled comparator group (one discontinued) |
Pertel et al25 | N=103 patients with cellulitis or erysipelas; aged ≥18 years | 4 mg/kg/day, 7–14 days | Vancomycin 1 g every 12 hours, 7–14 days | Clinical success rate: 94.0% with daptomycin and 90.2% with vancomycin (95% CI for the difference: 6.7%, 14.3%) | Similar incidences of AEs: 16.0% with daptomycin and 15.7% with vancomycin. AEs related to the study drug: three patients with daptomycin and one patient with vancomycin. No elevation of CPK was found |
Katz et al26 | N=100 patients with cSSTIs; aged ≥18 years | 10 mg/kg/day, 4 days | Vancomycin 1 g every 12 hours, up to 14 days | Clinical success rate: 75.0% with daptomycin and 87.5% with vancomycin (95% CI for the difference: −27.9%, 2.9%) | Similar incidences of AEs: 56.3% with daptomycin and 52.1% with vancomycin. AEs related to the study drug: 41.7% and 22.9%, respectively. Three patients had CPK elevations (>500 U/L) |
Fowler et al27 | N=246 patients with Staphylococcus aureus bacteremia with or without endocarditis; aged 21–91 years | 6 mg/kg/day, 14–15 days | Standard therapy: vancomycin 1 g every 12 hours with appropriate dose adjustment, or an anti-staphylococcal penicillin (nafcillin, oxacillin, or flucloxacillin) 2 g every 4 hours | Clinical success rate: 44.2% with daptomycin and 41.7% with standard therapy; absolute difference: 2.4% (95% CI for the difference: −10.2%, 15.1%) | Similar overall incidence of AEs in the two treatment groups. AEs related to the study drug: 42 (35.0%) with daptomycin and 49 (42.2%) with standard therapy. CPK elevations (>500 U/L): 9.5% and 1.5%, respectively, P=0.02 |
Arbeit et al28 | N=1,092 patients with cSSTIs; aged 18–85 years | 4 mg/kg/day, 7–14 days | Penicillinase-resistant penicillin 4–12 g four times a day or vancomycin 1 g every 12 hours by 60-minute infusion | Clinical success rate: 83.4% with daptomycin and 84.2% with comparators. No differences according to infection type (abscess, wound, ulcer) and infecting agent | Similar incidences of AEs between treatment groups. AEs related to the study drug: 94 (18%) with daptomycin and 119 (21%) with comparators. Discontinuation rates: 2.8% in both groups. CPK levels reported as drug-related AEs: 11 (2.1%) patients in the daptomycin and eight (1.4%) patients in the comparator group |
Abbreviations: AEs, adverse events; CI, confidence interval; CPK, creatine phosphokinase; cSSTIs, complicated skin and soft tissue infections.
Post-marketing real-world studies (mainly from large US [CORE] and European [EU-CORE] registries) support the safety of daptomycin and have shown similar findings as the clinical trials.29–35 A recent systematic review and meta-analysis36 showed that the overall incidence of treatment-related AEs was similar between daptomycin and comparator therapy groups. A significantly lower incidence of renal impairment, nausea, and headache was observed in the daptomycin therapy group. Elevated creatine phosphokinase (CPK) levels were reported, but these resolved rapidly after the discontinuation of daptomycin therapy. CPK elevation and associated skeletal muscle toxicity were frequently reported in early clinical studies that used multiple daily injections of daptomycin. The use of once-daily injection of daptomycin reduced the risk of this toxicity.2 The results from the two clinical Phase III trials showed that elevation in CPK was low (2.1% with daptomycin and 1.4% with standard treatment).28 Only two patients discontinued daptomycin; one had CPK elevation, and the second had symptoms of muscle toxicity. In another study, patients treated with daptomycin (6 mg/kg/day) for endocarditis and bacteremia experienced significantly more CPK elevation compared to standard treatment (6.7% vs 0.9%, P=0.04).27 However, only three patients discontinued daptomycin. The results from real-world clinical experience have shown that a small proportion of patients experienced serum CPK elevation (<2%) and severe skeletal muscle toxicity (0.1%).29
As shown in a few studies, high-dose daptomycin may elevate CPK level at an incidence of 2.5%–8.3%.26,37,38 However, CPK elevation during daptomycin therapy is not always associated with muscle toxicity.39–42 In healthy volunteers, doses up to 12 mg/kg/day were not associated with muscle toxicity.8 In a study on patients treated with high-dose (>6 mg/kg/day) daptomycin, CPK elevations were low (<3.0%).43 Concomitant use of daptomycin and statins may increase CPK levels. There was a twofold risk of CPK elevation with concurrent daptomycin and statin therapy as compared to daptomycin alone.44 The safety analysis of high-dose daptomycin showed similar rates of CPK elevation in those who received concomitant statin therapy (8%) as compared to those who did not (10%).45 CPK levels should be monitored weekly, or more often, in patients with myalgia or concomitant renal failure, or when drugs associated with elevated CPK levels and myopathy are coadministered.46
Daptomycin-induced acute eosinophilic pneumonia is a very rare, unpredictable, and potentially serious AE. It should be suspected in the context of fever, hypoxia, and pulmonary infiltrates.47,48 These symptoms resolve following the discontinuation of daptomycin; however, supportive therapy, including corticosteroids, may be required.49
Although not considered as an AE, apparent prolongation of the prothrombin time may be observed in patients receiving daptomycin due to an interaction with some test reagents, potentially leading to difficulties in therapeutic monitoring for anticoagulation therapy.50
Efficacy studies of daptomycin
Efficacy of daptomycin in patients with cSSTIs, bacteremia, and infective endocarditis caused by S. aureus was demonstrated in several randomized, controlled clinical trials (Table 2). Daptomycin (4 mg/kg/day) was compared in two randomized double-blind trials with vancomycin or penicillinase-resistant penicillin for the treatment of cSSTIs.28 Among 902 evaluated patients, the clinical efficacy of daptomycin was 83.4% as compared with 84.2% in the comparator group, and daptomycin required a shorter duration of administration in patients who were successfully treated with intravenous therapy alone. In another study designed to evaluate the efficacy of daptomycin at 6 mg/kg/day in patients with bacteremia or infective endocarditis caused by S. aureus, daptomycin was non-inferior to standard treatment (either vancomycin or an anti-staphylococcal penicillin).27 At the request of the Committee for Medicinal Products for Human Use, a comparative study of daptomycin in the elderly population was conducted, which showed that daptomycin had similar efficacy to semisynthetic penicillins and vancomycin in the treatment of cSSTIs.23 A recent meta-analysis including 13 randomized controlled trials compared the efficacy of daptomycin with that of comparator therapy.36 The results showed that daptomycin was as efficacious as standard therapy in the eradication of pathogens (methicillin-susceptible S. aureus, MRSA, Streptococcus pyogenes, Enterococcus faecalis, and Streptococcus pneumoniae), and significantly reduced the intravenous treatment duration.
Clinical trials with rigorous inclusion and exclusion criteria may not reflect true clinical experience. The EU-CORE study provided insight into the real-world clinical experience and supported findings from these clinical trials.29 Analyses from the EU-CORE study in patients with various Gram-positive infections (cSSTIs, bacteremia, right- and left-sided infective endocarditis, enterococcal infections, foreign body/prosthetic infections, and osteomyelitis) showed high and consistent rates of effectiveness.29–32,34,35
High-dose daptomycin
In difficult-to-treat infections, on the basis of its pharmacokinetic profile and concentration-dependent bactericidal activity, high-dose (>6 mg/kg/day) daptomycin may be considered due to the potential for more rapid bacterial clearance and reduced risk of emergence of resistance.51,52 The IDSA MRSA guidelines recommend consideration of high-dose (10 mg/kg/day) daptomycin in patients with persistent MRSA bacteremia associated with vancomycin failure.16 Several other national and international treatment guidelines include high-dose (8–10 mg/kg/day) daptomycin as a therapeutic option for difficult-to-treat infections including endocarditis, bacteremia, and bone/joint infection.53–55 High-dose daptomycin may also be advantageous in patients with sepsis and high volumes of distribution, or when there is a difficulty in achieving adequate local antibiotic concentration at the infection site.56,57
Several studies have suggested that higher doses (>6 mg/kg/day) of daptomycin are safe and effective in patients with bacteremia, osteomyelitis, foreign body/prosthetic infection (mainly orthopedic, intracardiac, and intravascular devices), and endocarditis (Table 3).26,38,43,58–60
Table 3
Clinical outcome and safety in high-dose daptomycin studies
Authors | Patients | Daptomycin (dose, treatment duration) | Comparator (type, dose, treatment duration) | Efficacy results | Safety results |
Katz et al26 | N=100 patients with cSSTIs; aged ≥18 years | 10 mg/kg/day, 4 days | Vancomycin 1 g every 12 hours, up to 14 days | Clinical success rate: 75.0% with daptomycin and 87.5% with comparator (95% CI for the difference: −27.9%, 2.9%) | Similar incidences of AEs: 56.3% with daptomycin group and 52.1% with vancomycin. AEs related to the study drug: 41.7% and 22.9%, respectively. Three patients had CPK elevations (>500 U/L) |
Moise et al38 | N=94 patients mainly with bacteremia, osteomyelitis, and endocarditis; aged ≥18 years | 8–10 mg/kg/day, 15 days | None | Overall clinical success rate was 89%, with the lowest success rate seen in patients with endocarditis | 29.8% of patients reported at least one AE or exhibited at least one abnormal laboratory result, and 6.4% of patients reported AEs as possibly related to daptomycin |
Seaton et al43 | N=1,097 patients mainly with osteomyelitis, foreign body/prosthetic infection, and endocarditis; aged ≥18 years | >6 mg/kg/day, 13–14 days | None | Overall clinical success rate was 81.9% | Daptomycin was well tolerated with no new or unexpected safety findings. Increased CPK was reported in 27 patients |
Byren et al58 | N=75 patients with osteomyelitis; aged ≥18 years | 6 and 8 mg/kg/day, 42 days | Standard-of-care antibiotics (vancomycin, teicoplanin, or semisynthetic penicillin), 40 days | Clinical success rates were 58.3% for 6 mg/kg/day daptomycin, 60.9% for 8 mg/kg daptomycin, and 38.1% for the comparator | No differences in the incidence of serious AEs across groups. CPK elevation of >500 U/L with daptomycin occurred in four (6 mg/kg/day) and five patients (8 mg/kg/day), and with comparator in two patients |
Carugati et al59 | N=178 patients with left-sided infective endocarditis; aged ≥18 years | 7.7–10 mg/kg/day, 39 days | Standard-of-care antibiotics (anti-staphylococcal penicillins, vancomycin, or ampicillin plus an aminoglycoside) | In-hospital mortality (primary outcome) was similar in both groups. Median time to clearance of methicillin-resistant Staphylococcus aureus bacteremia was 1.0 and 5.0 days (P<0.01) with daptomycin and standard-of-care antibiotics, respectively | Regimens with higher daptomycin doses were not associated with increased incidence of AEs |
Casapao et al60 | N=245 patients with enterococcal infections; aged ≥18 years | 7.7–9.7 mg/kg/day, 10 days | None | Overall clinical success rate was 89% | No patients experienced AEs attributed to high- dose daptomycin therapy. Seven patients had CPK elevations, yet no high-dose daptomycin regimen was discontinued due to an elevated CPK |
Abbreviations: AEs, adverse events; CI, confidence interval; CPK, creatine phosphokinase; cSSTIs, complicated skin and soft tissue infections.
Clinicians should be cautious when using non-licensed doses of daptomycin in obese patients as they may achieve higher exposure from reduced volume of distribution when compared to nonobese patients.18,61
Daptomycin within combination antimicrobial therapy
In clinical practice, daptomycin is recommended at doses that are higher than those currently approved (4–6 mg/kg/day) for the treatment of certain infections (osteomyelitis, foreign body/prosthetic infections, and enterococcal infections).16 However, emerging reports of the development of daptomycin resistance62 during therapy are cause of concern, and it may be appropriate to consider combination antibiotic therapy in patients at higher risk of developing daptomycin resistance.63 Interactions between daptomycin and other antibiotics have been studied in vitro, and it has been shown that activity of gentamicin and rifampicin administered concomitantly with daptomycin was not affected.64–67 Other in vitro models demonstrated that the combination of daptomycin and linezolid was synergistic and bactericidal for MRSA and for enterococci.68–70 In an in vitro simulated endocarditis pharmacokinetic/pharmacodynamic model, with daptomycin-nonsusceptible MRSA isolates (SA-684 and R6003), daptomycin plus trimethoprim–sulfamethoxazole was bactericidal (8 hours) and superior to daptomycin alone between 8 and 72 hours (P<0.001).71 In a clinical study, the overall clinical outcome was slightly enhanced with the addition of a β-lactam; this trend was better for bacteremia associated with endocarditis or bone/joint infection.72
Despite appropriate antimicrobial therapy, bacteremia due to MRSA remains a challenge.73 To increase activity and to prevent resistance, high-dose daptomycin used concomitantly with other antimicrobial agents has been considered in treating severe infections and is recommended within IDSA guidelines.16 Daptomycin combined with β-lactams prevents the emergence of resistance to daptomycin in clinical MRSA isolates and in enterococci.64,74–76 Mechanistically, β-lactams increase the negative charge of the bacterial cell surface leading to a better daptomycin binding and therefore improving its bactericidal activity.77 The synergy between daptomycin and β-lactams leading to bacterial killing and growth inhibition could also be explained by the penicillin-binding protein 1 inactivation following exposure to β-lactams.78,79 Daptomycin has also been used in combination with rifampin, trimethoprim–sulfamethoxazole, fosfomycin, tigecycline, and linezolid in order to achieve the resolution of MRSA infections.80–83 A combination of high-dose daptomycin and fosfomycin may be effective in the treatment of both native- and prosthetic-valve endocarditis caused by methicillin-susceptible S. aureus or MRSA.84
Daptomycin use in pediatric patients
Daptomycin is currently not approved for use in the pediatric population, and appropriate dosages for pediatric patients of different ages are yet to be clearly defined.85 A pharmacokinetic study in 25 children (2–17 years of age) receiving 4 mg/kg/day of daptomycin showed a more rapid clearance in those younger than 6 years.86 Another study conducted later showed that use of high-dose (8–10 mg/kg/day) daptomycin in children aged 2–6 years provided systemic exposure comparable to that seen in adults treated with the approved daptomycin doses of 4–6 mg/kg/day.87 Daptomycin’s role in pediatric Gram-positive infections has been evaluated in several studies, and a good safety profile has been observed.9,10,86–89 The IDSA MRSA treatment guidelines recommend the use of daptomycin (6–10 mg/kg/day) for managing MRSA bacteremia, infective endocarditis, acute hematogenous osteomyelitis, and septic arthritis in pediatric patients.16
In a recent multicenter, randomized, Phase III trial that included in total 396 children with cSSTIs, daptomycin given at age-appropriate doses was shown to be efficacious, safe, and generally well tolerated compared with the standard of care.15 Results from the EU-CORE registry showed that children and adolescent patients with a variety of Gram-positive infections treated with daptomycin had a high clinical success rate when daptomycin was used as a first- or second-line therapy.90
To further investigate the safety and efficacy of daptomycin in pediatric patients aged 1–17 years, a study (NCT01728376) comparing daptomycin to the standard of care for the treatment of S. aureus bacteremia was recently completed, and another study (NCT01922011) comparing daptomycin to vancomycin or nafcillin for the treatment of acute hematogenous osteomyelitis is currently ongoing.
Daptomycin use in outpatient practice
OPAT has been used in many countries to facilitate the cost-effective, safe administration of antibiotics as an alternative to an extensive and expensive hospital stay,91,92 and to reduce health care-associated infection risk and improve patient satisfaction.93,94 The most frequently treated infections in OPAT programs are skin and soft tissue infections (cellulitis, erysipelas, wound infection, and bursitis) and bone and joint infections (discitis, septic arthritis, diabetic foot osteomyelitis, and prosthetic joint and other metalwork-related infections).95 Careful attention to risk assessment and management can minimize potential risks in the OPAT setting,96 and several national guidelines have been developed to guide service development and patient management.93,97–99 Many studies have demonstrated that OPAT is indeed an effective and safe service.100–105 The clinical efficacy of OPAT has also been demonstrated for endocarditis or S. aureus bacteremia when delivered through a formal service model,106,107 and OPAT is now included in European, UK, and US guidelines on the management of endocarditis.108
The pharmacokinetic properties of daptomycin allow convenient once-daily 2-minute intravenous injection,6 which facilitates outpatient or home administration.95,109 This avoids multiple dosing or continuous infusion as required with other available antibiotics. Daptomycin in the OPAT setting is also associated with significantly fewer AEs and antimicrobial interventions compared to vancomycin.110 In EU-CORE, 12% of patients received daptomycin within an OPAT setting with an overall clinical success in 89% of these patients; the highest clinical success rates were in patients with bacteremia or endocarditis.95 Because of its ease of administration and an overall good safety profile, daptomycin has been considered as a first-line agent for use within OPAT.111–113
Daptomycin resistance
Emergence of antibiotic resistance in Gram-positive pathogens has become a major clinical and public health problem worldwide.78,114–116 Considering the limited number of new antimicrobial agents, the use of antibiotics in hospitals or elsewhere demands an acute awareness of the increasing problems with resistant organisms.5,62 According to the antimicrobial resistance global report on surveillance, antimicrobial resistance has been a growing threat to the effective treatment of an ever-increasing range of bacterial infections.117 To assess and monitor the magnitude and trends of the antibiotic resistance problem, major surveillance systems have been implemented.118
Daptomycin resistance has been previously documented for S. aureus and enterococci.119–123 Also, few clinical reports showing emergence of daptomycin resistance have been published until now. The prevalence of de novo resistance to daptomycin without prior exposure has been reported to be extremely rare, showing that only 0.04% among 10,000 S. aureus isolates tested had an MIC of 2 µg/mL.124 The Worldwide Surveillance Programme reported resistance data on daptomycin and numerous comparator agents in 164,457 Gram-positive clinical isolates (S. aureus, coagulase-negative staphylococci, enterococci, hemolytic streptococci) across five continents between 2005 and 2012. The results indicated that daptomycin remained potent against these indicated pathogens.125
The mechanisms of daptomycin resistance are multifactorial.74,116,126–128 Perturbation of the bacterial cell membrane and overexpression of dltA seem to be considered as the most common factors of resistance.124 This dysregulation of dltA transcription may result in a change of the bacterial cell membrane fluidity and therefore lead to a reduced affinity of daptomycin to its target site.129 The resistance pathways may vary among Gram-positive organisms.130 Mutations in the genes involved in phospholipid synthesis seem to be associated with the development of daptomycin resistance in S. aureus and E. faecalis.130,131
Future directions
Further clinical trials are warranted to better understand how to use high-dose daptomycin, that is, in which patient groups and for which pathogens, and to investigate the utility of short-course regimen. Such trials could provide data on how to minimize the risk of development of resistance and to optimize clinical outcomes. Also, understanding the optimal dosing of daptomycin remains a clinical priority, as well as increasing awareness of the relative benefits of combination therapy. Further research is needed in the form of well-designed, adequately powered trials comparing the efficacy and safety of daptomycin with other new anti-Gram-positive agents for treating different infections and eradicating pathogens.
Prosthetic joint infection is a devastating and costly complication of arthroplasty. Antibiotic-impregnated cement spacers are a useful tool for facilitating the penetration of the drug into the local infected area.132 The first clinical use of daptomycin-impregnated cement was described by Cortes et al who demonstrated a successful treatment in one patient with multiple allergies treated for chronic MRSA hip prosthetic infection.133 However, clinical data are lacking, and studies are needed to further evaluate daptomycin’s utility in this setting. More generally, implant-associated infection is serious and costly and associated with high morbidity.134 The presence of biofilm allows persistence of bacteria in a difficult-to-eradicate physiological state and increases the risk of resistance development.135 Further investigation of daptomycin’s role within biofilm-related infection is warranted.136
Conclusion
Daptomycin exhibits linear pharmacokinetics at doses up to 12 mg/kg/day and has been shown to be safe and efficacious for the treatment of cSSTIs, bacteremia, and RIE caused by susceptible Gram-positive bacteria in adults. In addition, according to post-marketing studies, daptomycin is a valuable treatment option in the management of other Gram-positive and difficult-to-treat infections, including left-sided endocarditis, osteomyelitis, prosthetic infections, and enterococcal infections.
On the basis of its pharmacokinetic profile and dose-dependent bactericidal activity, higher doses of daptomycin may be beneficial in treating severe infections. High-dose (>6 mg/kg/day) daptomycin used in the post-marketing observational studies exhibited a good safety and tolerability profile.
Of particular interest, daptomycin given in combination with other antibiotics may lead to resolution of various complex and resistant Gram-positive infections and may have a role (along with high-dose therapy) in minimizing the development of resistance.
Daptomycin appears promising as a safe and efficacious drug for the treatment of pediatric diseases caused by Gram-positive pathogens.
Daptomycin is recognized as an important agent in OPAT practice due to its spectrum of activity and ease of administration.
Although daptomycin resistance has been documented, it remains uncommon.
Acknowledgments
Medical writing support was provided by Farid Khalfi (Novartis Ireland Ltd, Dublin, Ireland).
Footnotes
Disclosure
AGR received fees from Novartis, Pfizer, Cubist, and Gilead for staff training, and is a member of advisory boards and speaker panels. RAS received consultancy fees and honoraria for speaking at Novartis-sponsored symposia. KH is an employee of Novartis Pharmaceuticals Corporation. The authors report no other conflicts of interest in this work.