

 2021-09-02
2021-09-02
ABSTRACT
In the face of increasing resistance to currently available antibiotics, there is a continued interest in the development of new drugs to treat Gram-positive infections. One such agent is the cyclic lipopeptide daptomycin—licensed in the USA for treatment of Gram-positive complicated skin and skin structure infections (cSSSIs) in 2003 and currently awaiting European approval for a similar indication (complicated skin and soft tissue infections). Daptomycin exerts its rapid bactericidal effect through insertion into and subsequent depolarisation of the bacterial cell membrane, a mode of action unlike that of any other available antibiotic. This novel mechanism of action makes the development of cross-resistance between daptomycin and other antibiotic classes unlikely. Daptomycin is highly active in vitro against a range of Gram-positive pathogens, including both susceptible and multidrug-resistant staphylococci and enterococci. Bactericidal activity has also been demonstrated against both growing and stationary-phase organisms, suggesting potential utility in the treatment of deep-seated infections. Two pivotal clinical studies comparing daptomycin 4 mg/kg per day intravenously with vancomycin or oxacillin-class antibiotics demonstrated the efficacy of daptomycin for treatment of cSSSIs. Daptomycin was well tolerated, with most adverse events considered to be unrelated to study medication, of mild-to-moderate intensity, and with a frequency and distribution similar to those associated with comparator antibiotics. The favourable clinical profile and low potential for development of resistance combine to make daptomycin a promising alternative to current agents for treatment of Gram-positive bacterial infections.
· Next article in issue
Keywords
Antibiotic
bactericidal
daptomycin
methicillin-resistant Staphylococcus aureus
review
vancomycin-resistant enterococci
vancomycin-resistant Staphylococcus aureus
INTRODUCTION
The number of cases of hospital-onset Staphylococcus aureus and enterococcal infections that are resistant to antimicrobial agents has increased significantly over the past three decades [
1,
2,
3]. Moreover, methicillin-resistant S. aureus (MRSA) is fast becoming a community-based as well as a hospital-based problem [
4,
5,
6,
7,
8]. The glycopeptidevancomycin has been the principal drug of choice for the treatment of MRSA infection for many years. However, over-reliance on vancomycin has resulted in the recent development of the first cases of resistance [
9,
10,
11,
12], and therefore its continued clinical value may be expected to become self-limiting. Other agents currently used to treat MRSA infection include the glycopeptide teicoplanin, the streptogramin combination quinupristin–dalfopristin, and the oxazolidinonelinezolid. The clinical value of each of these treatments is, however, limited by one or more factors, including antibacterial spectrum of activity, complex administration, side-effect profile and the increasing emergence of resistant strains [1,
13,
14,
15,
16]. Not surprisingly, therefore, the development of new antibacterial compounds to combat Gram-positive infections continues to be a prime area of drug research. Indeed, several compounds are in development, including ceftobiprole, dalbavancin, daptomycin, oritavancin, telavancin and tigecycline. However, many developmental antibiotics are based on existing classes of antimicrobial agents, and therefore raises concern, at least theoretically, that antibiotic resistance will develop quickly, notably through cross-resistance with existing agents.
Daptomycin is the first of a novel group of compounds classified as the cyclic lipopeptides. This article will review the mechanism of action of daptomycin, and its pharmacokinetics, efficacy against Gram-positive bacteria (particularly staphylococci) and potential for leading to the development of resistance. Clinical trial efficacy and safety data will also be presented to assess the potential role of daptomycin as a treatment against Gram-positive pathogens.
DEVELOPMENT OF DAPTOMYCIN
Daptomycin was first isolated from Streptomyces roseosporus through a soil-screening programme, and clinical development by Eli Lilly began in 1985. The initial development programme was terminated with the occurrence of potential drug-induced myopathic events in two subjects during phase I clinical trials. Over time, however, as understanding of the occurrence, effects and management of drug-induced myopathies increased, and as the need for new antibiotics—particularly those with activity against MRSA—became more pressing, the potential clinical utility of daptomycin was re-evaluated. Cubist Pharmaceuticals Inc. licensed daptomycin from Lilly in 1997 and re-instigated clinical development using a refined once-daily dosing regimen, with the aim of retaining antimicrobial efficacy while minimising potential toxicity. Chiron BioPharmaceuticals, under licence from Cubist, is developing the drug in Europe and various other regions, excluding the USA. Daptomycin has been in clinical use in the USA since 2003 for the treatment of Gram-positive complicated skin and skin structure infections (cSSSIs), and European approval of its use for Gram-positive complicated skin and soft tissue infections is expected in early 2006.
MECHANISM OF ACTION
Daptomycin has a mode of action unlike that of any other available antibiotic to date [
17]. It is a 13-membered amino-acid cyclic lipopeptide with a hydrophilic core and hydrophobic tail [17]. The hydrophobic tail of daptomycin binds irreversibly to the cell membrane of Gram-positive bacteria via a calcium-dependent process [17,
18,
19]. A channel is formed, causing rapid depolarisation of the cell membrane due to efflux of potassium, and possibly other cytoplasmic ions [17]. Bacterial cell death results from the widespread dysfunction of macromolecular synthesis [17, 19]. Unlike β-lactam antibiotics, daptomycin does not depend on cell lysis to kill the bacterial cell [19].
PHARMACOKINETICS
Daptomycin has a relatively long half-life of 8–9 h, thereby making it suitable for once-daily dosing [
20]. It exhibits consistent and predictable kinetics for doses of 4, 6 and 8 mg/kg per day (maximum concentration (Cmax) of 58, 99 and 133 mg/L, 24-h area under the curve (AUC) of 494, 747 and 1130 mg/h per litre, respectively) [20]. Its low volume of distribution (0.1 L/kg) indicates that it remains primarily within plasma and interstitial fluid [20]. Daptomycin is excreted mainly in urine (78%), with approximately 50% of the active drug being recovered unchanged from urine within 24 h [20,
21]. A small proportion of daptomycin (6%) is also recovered in faeces.
Daptomycin exhibits approximately 92% binding to plasma proteins, especially albumin. However, its binding to plasma proteins appears to be weaker than the irreversible bond it forms with the bacterial membrane, resulting in daptomycin being significantly more bioavailable than this level of protein binding would suggest [
22]. Indeed, one study, presented at the 42nd Infectious Diseases Society of America Annual Meeting, 30 September – 3 October 2004, demonstrated that the extent of increase in MICs of daptomycin in 5% albumin and 95% human and mouse sera was less than would be expected from its level of protein binding (abstract 302). Another study, presented at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC (16–19 December 2005), reported only 4.44- to 5.33-fold increases in MIC of daptomycin in the presence of 4% human albumin, 50% human serum or 100% mouse serum (abstract D-1644). These data suggest that daptomycin is two- to four-fold more active than predicted from its free unbound drug concentrations.
AUC/MIC or Cmax/MIC pharmacodynamic ratios best correlate with in-vivo efficacy against standard strains of S. aureus and Streptococcus pneumoniae [
23]. Given the fact that daptomycin neither inhibits nor stimulates cytochrome P450 enzymes [21,
24], and that there are currently no known drug–drug interactions, daptomycin may be used in combination with a range of other therapeutic agents.
IN-VITRO EFFICACY
Daptomycin has demonstrated broad efficacy against Gram-positive pathogens, including susceptible and multidrug-resistant staphylococci and enterococci. Its activity has been compared in vitro with those of vancomycin, linezolid and quinupristin–dalfopristin for a range of Gram-positive bacterial strains (n = 203), including MRSA, vancomycin-resistant enterococci and vancomycin-intermediate S. aureus [
25,
26]. The MICs at which 90% of strains were inhibited are shown in
Table 1. Overall, daptomycin was more active against all organisms tested except Enterococcus faecium, against which its activity was similar to that of quinupristin–dalfopristin. Other studies showed that daptomycin is active against most of the clinical isolates tested, with MICs between 0.006 and 2.0 mg/L [
27,
28,
29,
30,
31,
32]. Furthermore, in a worldwide surveillance study, daptomycin was shown to be active against all strains of vancomycin-resistant E. faecium (MIC90 4 mg/L) [
33].
Table 1. Activities of daptomycin and other antibiotics against various bacterial strainsin vitro[25]
Bacteria (no. of isolates) | MIC90 mg/L | |||
Daptomycin | Vancomycin | Linezolid | Quinopristin–dalfopristin | |
MSSA (50) | 0.13 | 1.0 | 4.0 | 1.0 |
MRSA (50) | 0.13 | 1.0 | 4.0 | 1.0 |
MSSE (25) | 0.50 | 1.0 | 4.0 | 0.50 |
MRSE (25) | 0.25 | 1.0 | 4.0 | 0.25 |
href="#tbl1fn1" a | 1.0 | 64.0 | 4.0 | 16.0 |
Enterococcus faecium (25)a | 4.0 | 64.0 | 4.0 | 4.0 |
MIC90, minimum inhibitory concentration at which 90% of strains are inhibited; MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis; MSSA, methicillin-susceptible Staphylococcus epidermidis; MSSE, methicillin-susceptible Staphylococcus epidermidis.
a
Includes vancomycin-resistant enterococci.
Notably, daptomycin is also active in vitro against the recently isolated Michigan and Pennsylvania (Hershey) strains of vancomycin-resistant S. aureus (MICs of 1.0 and 0.5 mg/L, respectively) [10, 27,
34].
BACTERICIDAL ACTIVITY
Daptomycin is rapidly bactericidal in vitro [17]. In time-kill studies, at a concentration of four times the MIC, daptomycin achieved 99.9% killing of MRSA in 8 h, which was greater than the kill rates for either linezolid or quinupristin–dalfopristin (p < 0.05) [25]. In a comparison of four antibiotics, daptomycin, at a concentration of 2 mg/L (the tentative breakpoint at the time of the study), resulted in bactericidal activity against 92% of strains tested (108 staphylococcal isolates, including MRSA and vancomycin-intermediate S. aureus) [
35]; the other rates found were 72% for vancomycin, 46% for quinupristin–dalfopristin and 7% for linezolid at their respective breakpoint concentrations.
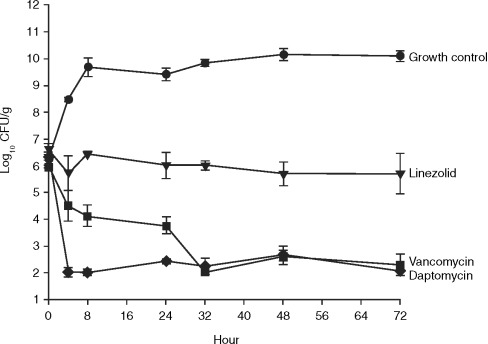
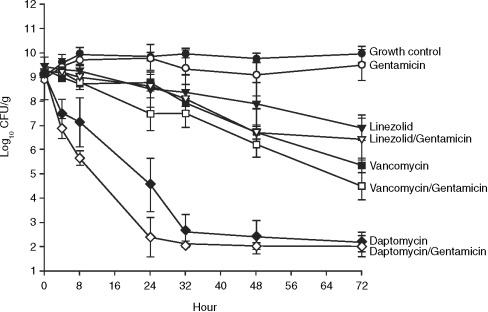
Sequestered high-bacterial-density infections, such as those found in patients with endocarditis and osteomyelitis, are often difficult to treat. Antibiotic failure may occur because of poor drug penetration, inoculum effects, high degree of protein binding, and the presence of stationary-phase organisms—a likely consequence of nutrient limitations at the seat of the infection [17,
36]. In a comparison of daptomycin, nafcillin, linezolid and vancomycin, the activities of the antibiotics were investigated alone, and in combination with gentamicin, against high (9.5 log10 CFU/g) and moderate (5.5 log10 CFU/g) inocula of methicillin-susceptible S. aureus (MSSA) and MRSA over 72 h in an in-vitro pharmacodynamic model simulating endocardial vegetations [36]. Human therapeutic dosing regimens of each drug were simulated. Comparable bactericidal activities (99.9% kill) were seen for nafcillin (MSSA only), vancomycin and daptomycin against a moderate inocolum, with a more rapid onset of activity for daptomycin and nafcillin than for vancomycin (4 vs. 32 h, respectively (
Fig. 1). At a high inoculum, daptomycin demonstrated bactericidal activity against both MSSA and MRSA by 24 h, whereas vancomycin, linezolid (against both MSSA and MRSA) and nafcillin (against MSSA alone) did not achieve bactericidal activity throughout the entire 72-h study period (
Fig. 2). The addition of gentamicin increased the rate of bactericidal activity of daptomycin to 8 h and that of nafcillin to 48 h, but had no effect on the rates of kill for either vancomycin or linezolid over the duration of the experiment. Furthermore, daptomycin's bactericidal activity, unlike most other agents, has been shown to be maintained in the stationary phase, which may be an important factor in the treatment of deep-seated infections [
37].
1. Download : Download full-size image
Fig. 1. In-vitro bactericidal activity of daptomycin, linezolid and vancomycin against a moderate inoculum of methicillin-resistant Staphylococcus aureus [36].
1. Download : Download full-size image
Fig. 2. In-vitro bactericidal activity of daptomycin, linezolid, vancomycin and gentamicin (alone and in combination) against a high inoculum of methicillin-resistant Staphylococcus aureus [36].
The bactericidal effects of daptomycin have also been demonstrated using minimum bactericidal concentration/MIC ratio determination. Rybak et al. tested 103 S. aureus isolates (50 MRSA, 50 MSSA and three glycopeptide-intermediate S. aureus) and 50 Staphylococcus epidermidis isolates (25 methicillin-susceptible S. epidermidis and 25 methicillin-resistant S. epidermidis) and demonstrated the bactericidal activity of daptomycin (i.e., minimum bactericidal concentration within two dilutions of MIC) to be superior to that of vancomycin, linezolid or quinupristin–dalfopristin [25]. The minimum bactericidal concentration/MIC ratios of daptomycin for 17 E. faecium isolates (vancomycin-resistant) were determined by Snydman et al. [
38]. Daptomycin was bactericidal for 82% (14/17) of the strains.
SYNERGY STUDIES AND POST-ANTIBIOTIC EFFECT
The activity of daptomycin has also been investigated in combination with other antibiotics, most commonly against Enterococcus spp. Synergy was observed against 13 of 18 vancomycin-resistant enterococcal isolates treated with daptomycin plus rifampin using an agar Etest screening method; a later time-kill study supported these results [
39]. Additive or synergistic effects have also been demonstrated with daptomycin plus ampicillin against Enterococcus faecalis and E. faecium isolates as well as daptomycin plus gentamicin against an ampicillin-resistant E. faecium isolate [
40]. Antagonism between daptomycin and other antibiotics has not been seen.
Daptomycin has a long-lasting post-antibiotic effect, which is concentration-dependent and lasts up to 6 h against S. aureus and E. faecalis in the presence of free calcium at physiological concentrations [
41].
DEVELOPMENT OF RESISTANCE TO DAPTOMYCIN
History has shown that whenever a new antibiotic is introduced into widespread use, clinically significant resistance eventually appears. In-vitro studies have shown, to date, that it is difficult to generate daptomycin-resistant isolates. Daptomycin's unique mechanism of action targets the bacterial cell wall, and this is typically associated with a relatively slow rate of inheriting resistance compared with, for example, rRNA [17]. In-vitro experiments testing for the emergence of spontaneous resistance to daptomycin demonstrated that no spontaneous resistant mutants were obtained for any of the bacteria tested: < 10−10 for S. aureus, < 10−9 for S. epidermidis, E. faecalis or E. faecium, and < 10−8 for S. pneumoniae [
42]. Furthermore, over 20 passages in the presence of daptomycin were required to generate a modest number of isolates with reduced susceptibility to daptomycin. Stable S. aureus mutants could be generated by both serial passage in liquid media (one isolate in 27 days) and chemical mutagenesis (11 isolates), but these mutants had only modest (eight- to 32-fold) increases in daptomycin MICs compared with the parental strain [42]. Consistent with these in-vitro findings, the emergence of daptomycin resistance during clinical use has been rare, with only three instances in E. faecium (MIC > 32 mg/L), one in E. faecalis (MIC 16 mg/L) and five in S. aureus (MIC 2–8 mg/L) reported to date (45th Interscience Conference on Antimicrobial Agents and Chemotherapy, 16–19 December 2005, abstract L-2141; Infectious Disease Society of America 2005 Annual Meeting, 6–9 October 2005, abstract 490 [
43,
44,
45,
46,
47,
48]).
None of the daptomycin-resistant mutants generated in vitro have demonstrated resistance to vancomycin or ampicillin, which is consistent with the differences in the mechanisms of action of these drugs [42]. The development of cross-resistance between daptomycin and glycopeptide or β-lactam antibiotics appears unlikely because of daptomycin's unique mechanism of action [
49]. The problem of cross-resistance is common with many other antibiotics because they have been developed by making modifications to existing classes of antibiotics [49].
CLINICAL STUDIES COMPLICATED SKIN AND SOFT TISSUE INFECTIONS
Food and Drug Administration approval for the use of daptomycin to treat Gram-positive cSSSIs in the USA was obtained, based on the results yielded by two pivotal clinical studies. Each randomised, evaluator-blinded phase III study involved approximately 500 patients (total population 1092 patients, aged 18–85 years). Patients presented with cSSSIs, associated or potentially associated with Gram-positive bacteria, including abscesses, surgical and traumatic wound infections and infected diabetic foot ulcers [
50]. Daptomycin 4 mg/kg intravenously once-daily, administered as a 30-min infusion, was compared with vancomycin 1 g intravenously twice-daily, given as a 60-min infusion, or an oxacillin-class antibiotic (cloxacillin, flucloxacillin, oxacillin or nafcillin) 4–12 g intravenously once-daily, for 7–14 days. The primary objective of the study was to demonstrate that daptomycin was not inferior to the comparator agents. Non-inferiority was defined as an upper boundary of 95% CI of less than 10% (as recommended by the Division of Anti-Infective Drug Products of the US Food and Drug Administration).
Both trials (individually and collectively) met the clinical and statistical criteria for demonstrating non-inferiority of daptomycin to comparator antibiotic treatment; results were consistent across all predefined patient populations, different species of Gram-positive bacteria, all types of infection and post-treatment relapse rates [50]. For the combined intention-to-treat population (n = 1092), clinical success rates were 71.5% and 71.1% (95% CI –5.8 to 5.0) for daptomycin- and comparator-treated patients, respectively. Equivalent rates in the clinically evaluable population were 83.4% and 84.2%, respectively. The clinical success rates, broken down by infecting Gram-positive organism for the microbiologically evaluable population, are shown in
Table 2, and also demonstrate that treatment with daptomycin was not inferior to treatment with the comparator antibiotics. An interesting finding of this study was the shorter duration of treatment required with daptomycin than with comparator antibiotics, as decided by the study investigators (
Table 2. Clinical success rates for daptomycin and comparator treatment for complicated skin and skin structure infections, broken down by infecting Gram-positive organism for the microbiologically evaluable population [50]
Infective organism | Daptomycin | href="#tbl2fn1" a | 95% CI |
href="#tbl2fn2" b | |||
Methicillin-susceptible | 170/198 (85.9%) | 180/207 (87.0%) | − 5.6 to 7.8 |
Methicillin-resistant | 21/28 (75.0%) | 25/36 (69.4%) | − 28.5 to 17.4 |
Streptococcus pyogenes | 79/84 (94.0%) | 80/88 (90.9%) | − 11.1 to 4.9 |
Streptococcus agalactiae | 23/27 (85.2%) | 22/29 (75.9%) | − 30.9 to 12.2 |
Streptococcus dysgalactiae | 8/8 (100%) | 9/11 (81.8%) | − 48.6 to 12.2 |
Enterococcus faecalis | 27/37 (73.0%) | 40/53 (75.5%) | − 16.3 to 21.3 |
a
Cloxacillin, flucloxacillin, nafcillin, oxacillin or vancomycin.
b
Methicillin susceptibility was determined only for isolates received by the central microbiology laboratories.
Table 3. Duration of intravenous antibiotic therapy in patients successfully treated with intravenous daptomycin or comparator antibiotic for complicated skin and skin structure infections. Groups comprise those patients from the clinically evaluable (CE) population who were considered as clinical successes and who did not receive oral antibiotics [50]
Duration of treatment | Daptomycin (n = 372; 83.4% of CE) | href="#tbl3fn1" a (n = 384; 84.2% of CE) |
4–7 days | 63% | 33% |
≥ 8 days | 37% | 67% |
CE, clinically evaluable.
a
Cloxacillin, flucloxacillin, nafcillin, oxacillin or vancomycin.
Other clinical trials
Daptomycin is currently indicated in the USA for the treatment of cSSSIs caused by strains of Staph. aureus (including MRSA), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae equisimilis and E. faecalis (vancomycin-susceptible isolates only). In Europe, daptomycin is currently being reviewed by the European Medicines Evaluation Agency for the treatment of Gram-positive complicated skin and soft tissue infections. Daptomycin is also being investigated for other conditions. A recently completed international, multicentre, prospective, randomised, controlled, open-label phase III trial evaluated daptomycin in patients with S. aureusendocarditis or bacteraemia [17]. Patients with bacteraemia (MSSA or MRSA) were randomised for 2–6 weeks of treatment with intravenous daptomycin, 6 mg/kg daily, or a semi-synthetic intravenous penicillin, 2 g/6-times-daily, or intravenous vancomycin, at standard doses twice-daily, according to susceptibility. MRSA was isolated from 37% and 38% of patients in the daptomycin and comparator arms, respectively. Patients in the comparator arm of the study received an initial 4 days of treatment with intravenous gentamicin, and all patients were followed up for 12 weeks. The primary endpoint of non-inferiority was met in both the intention-to-treat (n = 235) and per-protocol (n = 139) populations. The most common adverse events in both the daptomycin and comparator arms included musculo-skeletal symptoms, nausea, vomiting and oedema. These data were presented as a late-breaker session at the Interscience Conference on Antimicrobial Agents and Chemotherapy, 16–19 December 2005, abstract K-426a.
The efficacy of daptomycin for the treatment of community-acquired pneumonia was investigated in two international phase III clinical trials including almost 1000 patients (Cubist Pharmaceuticals, data on file; reported by LaPlante and Rybak [
51]). The primary objective was to demonstrate non-inferiority in clinical efficacy (defined as resolution of signs and symptoms) of intravenous daptomycin 4 mg/kg per day compared to standard therapy (intravenousceftriaxone 2 g/day). However, the primary endpoint was not met, possibly as a consequence of the significant reduction in activity of daptomycin in the presence of pulmonary surfactant [
52]. Daptomycin is therefore not indicated for the treatment of pneumonia [21].
SAFETY AND TOLERABILITY
In the two pivotal phase III efficacy trials for cSSSIs, daptomycin was well-tolerated, with the frequency and distribution of adverse events (AEs) being similar for patients receiving daptomycin (n = 534) and those given comparator antibiotics (n = 558) [50]. Most AEs were considered to be unrelated to study medication and were of mild-to-moderate intensity. One or more drug-related AEs occurred in 18% of daptomycin-treated patients and in 21% of patients treated with comparator agents [50]. The most frequently experienced AEs were gastrointestinal disturbances, injection site reactions and headache, at frequencies comparableto orlower than those seen with comparator antibiotics [50]. A total of 2.8% of patients from each group discontinued treatment.
Given the potential for daptomycin-related muscle toxicity as reported in the early phase trials of daptomycin that employed multiple daily dosing, creatine phosphokinase (CPK) levels were closely monitored throughout the studies. The distribution of CPK values was comparable across treatment groups at baseline, as well as during and after treatment [50]. In the phase III cSSSI trials, elevations in CPK levels were reported in 9.3% of daptomycin recipients and 8.9% of patients treated with the comparator drug [
53]. Only two (0.2%) patients treated with daptomycin experienced CPK elevations associated with myalgia and/or muscle weakness. In both these cases, clinical symptoms as well as laboratory abnormalities resolved after discontinuation of treatment with daptomycin.
CONCLUSIONS
Daptomycin is the first member of a novel class of antibiotics, the cyclic lipopeptides. It has a rapid onset and broad spectrum of bactericidal activity against Gram-positive pathogens, including MRSA, vancomycin-intermediate S. aureus, vancomycin-resistant S. aureus and vancomycin-resistant enterococcal isolates. It is highly active against both growing and stationary-phase bacteria. In clinical trials, daptomycin demonstrated a favourable safety profile and efficacy comparable to that of standard therapy in the treatment of cSSSIs. Because of its unique mode of action, daptomycin has a low propensity to result in the development of resistant bacteria.
Daptomycin was introduced into clinical practice in the USA in 2003 and is currently indicated for the treatment of cSSSIs; it is expected to be available in Europe early in 2006. Results from recently completed and ongoing studies will establish the spectrum of clinical utility of daptomycin as a potential alternative to current agents, notably the glycopeptides, for the treatment of other Gram-positive bacterial infections.