

 2021-08-17
2021-08-17
Received: 11 January 2006 / Accepted: 13 February 2006 / Published online: 30 March 2007
§C Springer Science+Business Media, Inc. 2007
Background of patients. Two patients in the CP group were excluded from the study because their stool consistency was too firm and they experienced difficulty in defecating after
Table 1 Background of each group
CP Control
Mean age, yr (range) | 30.6 (20–56) | 33.1 (20–51) |
Males:females | 6:2 | 4:4 |
Two-stage:three-stage | ||
operations | 1:7 | 2:6 |
taking the drug. Another patient in the CP group and two patients in the control group were excluded from the study per the patients’ request. Eight patients were enrolled in each group; the mean age, sex distribution, and ratio of two- to three-stage operations did not differ between the groups (Table 1).
Anal manometry. MRP in the control and CP groups was
![]()
55 11 and 60 10 cm H2O, respectively, before drug ad- ministration and these values did not differ from each other (Fig. 1). Furthermore, the postdrug MRPs did not differ at 1 and 6 months from the values measured before drug ad- ministration in both groups. No statistical differences were observed between the control and the CP groups 1 or 6 months after drug administration (Fig. 1).
![]()
![]()
Bowel function. Stool frequency (events/day) before drug administration was 10.7 1.6 and 13.0 1.7 in the con- trol and CP groups, respectively (P > 0.05). However, stool frequency decreased in a time-dependent manner in both groups, yielding values 6 months postdrug of 6.8 1.0 and
9.1
![]()
![]()
![]()
1.3 per day in the control and CP groups, respec- tively (Fig. 2). Although stool frequency in the control group was reduced in comparison to that in the CP group, no statistical differences were noted (Fig. 2). There were no time-dependent changes in stool consistency in either group (Fig. 3). The mean score for nighttime soiling was 3.4 0.6 and 2.1 0.4 in the control and CP groups, respectively, prior to drug administration, and these values were not statistically
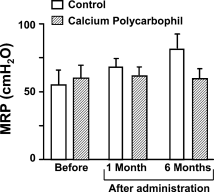
Fig. 1 Change in MRP. No time-dependent increase in MRP was observed in either the CP-treated or the control group. No differences were observed between the groups before or 1 and 3 months after drug administration.
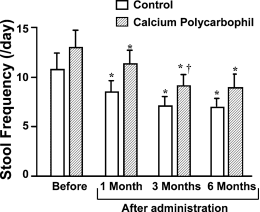
Fig. 2 Change in stool frequency. Stool frequenc
y decreased in a time-dependent manner after drug administration in both groups. Al- though stool frequency in the control group was low in comparison to that in the CP group, no statistical difference was identified across groups. ∗P < 0.05 compared to the value before drug administration in the same group. †P < 0.05 compared to the value 1 month after drug administration in the same group.
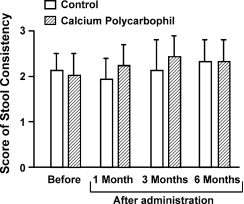
Fig. 3 Change in stool consistency. There was no time-dependent increase or decrease in the score for stool consistency in either group, and no two values were statistically different from each other.
![]()
![]()
different from one another (Fig. 4). These scores increased in a time-dependent manner, to 4.3 0.4 and 4.6 0.2 in the control and CP groups, respectively, after 6 months of drug administration. However, the time-dependent increase in score did not reach statistical difference in the control group (Fig. 4). Although the score at 1 month after CP ad- ministration decreased and was significantly lower than that measured in the control group, it increased thereafter in the CP group, i.e., after 6 months of CP, the score (4.6 0.2) was equivalent to that in the control group and higher than that in the CP group before and 1 month after drug administration (P < 0.05; Fig. 4).
Fig. 4 Change in score for nighttime soiling. Although the score tended to increase in a time-dependent manner, this was not obvious in the control group. The score for nighttime soiling 6 months after drug administration was significantly higher than that prior to and 1 month after drug administration in the CP group. ∗P < 0.05 compared to the value before drug administration in the same group. †P < 0.05 compared to the value 1 month after drug administration in the same group.‡ P < 0.05 compared to the value 1 month after drug administra- tion in the control group.
The effect of CP on MRP and stool frequency or consis- tency in patients undergoing proctocolectomy with IPAA was not obvious. However, patients receiving CP exhibited diminished nighttime soiling with time in comparison to the control group, suggesting that CP may ameliorate night- time soiling by hardening the stool. Any CP-induced im- provement in stool consistency may not have been sufficient to permit recognition by patients as a change in stool ap- pearance. It is noteworthy that two patients taking CP were excluded from the study due to difficulty in defecating asso- ciated with a too firm stool consistency. The process leading to improved bowel function after procotcolectomy is referred to as “intestinal adaptation.“ IPAA significantly increases the villus length and density and the mucosal surface area/serosal length ratio in the ileum [7]. A concomitant increase in the plasma concentration of peptides released from the ileum (e.g., peptide YY) may contribute to these morphological changes [8]. Stool consistency is related to the ratio of water retention by insoluble solids to total luminal water in the lu- men, with a low ratio being associated with diarrhea [5]. CP is a synthetic, high molecular weight polymer with marked water retention and gel-forming capacities [9]. The former property reduces constipation, whereas the gelling property ameliorates diarrhea. The laxative and antidiarrheic effects of CP have been reported in dogs and rodents [5, 9]. It is likely that the gelling property of CP may promote this adaptation by affecting the water content in stool.
The most popular antidiarrheic agent for chronic diar- rhea and postproctocolectomy diarrhea is loperamide [10,
11]. In patients with restorative proctocolectomy, oral lop- eramide is thought to diminish stool frequency by affecting the small intestine proximal to the pouch, since loperamide suppositories suppressed pouch contraction but did not re- duce stool frequency [1]. Others reported that loperamide reduced bowel frequency by diminishing total stool weight, but not individual stool weights, in patients with IPAA [12]. In a canine model of IPAA, the loperamide-induced decline in stool frequency was attributed to its antisecretory effects, because the drug did not affect motility in the small intestine and pouch [13]. The clinical studies employed loperamide doses which are substantially higher, i.e., 8–12 mg/day, than the doses used in Japan (2 mg/day). Thus, we cannot antici- pate the use of loperamide as an effective antidiarrheic agent for postcolectomy diarrhea at such low standard doses.
Michelassi et al. [14] followed long-term functional re- sults after IPAA for UC and reported that the number of bowel movements per day did not decrease when monitored at 3-month intervals extending beyond the initial 3 months after the surgery. Their data appear to conflict with our re- sults in the control and CP groups, i.e., stool frequency at 1, 3, and 6 months after drug administration was less in com- parison to the values recorded in the premedicated samples. However, our data demonstrating that stool frequency in the control and CP groups did not differ between 3 and 6 months post-drug administration do not conflict with the r
esults of Michelassi et al. Those authors also stated that the severity of incontinence declined with time, which was suggested as the decrease in the percentage of patients with major leakage. A similar phenomenon was observed in our CP group but not in the control group. Our results on nighttime soiling do not conflict with theirs, because patients taking antidiarrheic agents are included in their study. Although the score for nighttime soiling increased time dependently in the control group, the increase was not statistically significant. However, this trend in the control group must be associated with a sig- nificant difference in scores between the CP and the control groups 1 month after drug administration. It is also possible that the frequency of nighttime soiling would be reduced even in patients not taking antidiarrheic agents later
than 6 months after the surgery.
The small number of patients in our study is a limitation. However, we believe that eight patients in each group are enough for analysis. Statistical differences were observed in the time-dependent decrease in stool frequency (Fig. 2) and the time-dependent increase in the score for nighttime soiling in the CP group (Fig. 4). In conclusion, our results
suggest that CP may diminish the frequency of nighttime soiling after IPAA for UC. CP does not appear to alter either stool frequency or consistency.
Acknowledgments This study was supported by Abbott Japan Co., Ltd., Tokyo. The study was presented at the Annual Meeting of the Society for Surgery of the Alimentary Tract, 2005, in Chicago, Illinois.