

Tacrolimus
| Name | Tacrolimus |
| CAS No. | 104987-11-3 |
| Sepcification | USP42 |
| Usage and indications | It has similar immunosuppressive properties to ciclosporin, but is much more potent. Immunosuppression with tacrolimus was associated with a significantly lower rate of acute rejection compared with ciclosporin-based immunosuppression (30.7% vs 46.4%) in one study. Clinical outcome is better with tacrolimus than with ciclosporin during the first year of liver transplantation. Long-term outcome has not been improved to the same extent. Tacrolimus is normally prescribed as part of a post-transplant cocktail including steroids, mycophenolate, and IL-2 receptor inhibitors such as basiliximab. |
| Structural formaula | 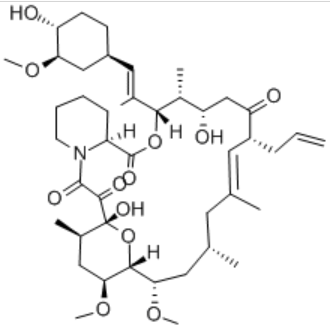 |
| Description | 1. Dosage form and Mechanism of action Dosage form: Capsule/Injection Mechanism of action: Tacrolimus is a macrolide calcineurin inhibitor. In T-cells, activation of the T-cell receptor normally increases intracellular calcium, which acts via calmodulin to activate calcineurin. Calcineurin then dephosphorylates the transcription factor nuclear factor of activated T-cells (NF-AT), which moves to the nucleus of the T-cell and increases the activity of genes coding for IL-2 and related cytokines. Tacrolimus prevents the dephosphorylation of NF-AT. In detail, tacrolimus reduces peptidylprolyl isomerase activity by binding to the immunophilin FKBP12 (FK506 binding protein), creating a new complex. This FKBP12–FK506 complex interacts with and inhibits calcineurin, thus inhibiting both T-lymphocyte signal transduction and IL-2 transcription. Although this activity is similar to that of cyclosporin, the incidence of acute rejection is reduced by tacrolimus use over cyclosporin use. Although short-term immunosuppression concerning patient and graft survival is found to be similar between the two drugs, tacrolimus results in a more favorable lipid profile, and this may have important long-term implications given the prognostic influence of rejection on graft survival. 2. Specification 3. Package 500 grams/bag; 1000 grams/bag; It can also be packed according to the requirements 4. Product source Produced by Chemical synthesis in China 5. Document support SMF and DMF are available 6. Supply Stable supply for more than 4 years If you need, Please contact us:info@shjnj.com |